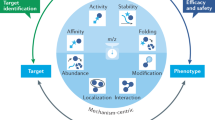
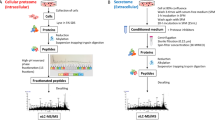
Abstract
Most therapeutics are designed to alter the activities of proteins. From metabolic enzymes to cell surface receptors, connecting the function of a protein to a cellular phenotype, to the activity of a drug and to a clinical outcome represents key mechanistic milestones during drug development. Yet, even for therapeutics with exquisite specificity, the sequence of events following target engagement can be complex. Interconnected communities of structural, metabolic and signalling proteins modulate diverse downstream effects that manifest as interindividual differences in efficacy, adverse effects and resistance to therapy. Recent advances in mass spectrometry proteomics have made it possible to decipher these complex relationships and to understand how factors such as genotype, cell type, local environment and external perturbations influence them. In this Review, we explore how proteomic technologies are expanding our understanding of protein communities and their responses to large- and small-molecule therapeutics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Milo, R. What is the total number of protein molecules per cell volume? A call to rethink some published values. Bioessays 35, 1050–1055 (2013).
Klijn, C. et al. A comprehensive transcriptional portrait of human cancer cell lines. Nat. Biotechnol. 33, 306–312 (2015).
Nagaraj, N. et al. Deep proteome and transcriptome mapping of a human cancer cell line. Mol. Syst. Biol. 7, 548 (2011).
Wilhelm, M. et al. Mass-spectrometry-based draft of the human proteome. Nature 509, 582–587 (2014).
Kim, M.-S. et al. A draft map of the human proteome. Nature 509, 575–581 (2014).
Bekker-Jensen, D. B. et al. An optimized shotgun strategy for the rapid generation of comprehensive human proteomes. Cell Syst. 4, 587–599 (2017).
Wis´niewski, J. R., Hein, M. Y., Cox, J. & Mann, M. A ‘proteomic ruler’ for protein copy number and concentration estimation without spike-in standards. Mol. Cell. Proteom. 13, 3497–3506 (2014).
Cooper, H. L., Park, M. H. & Folk, J. E. Posttranslational formation of hypusine in a single major protein occurs generally in growing cells and is associated with activation of lymphocyte growth. Cell 29, 791–797 (1982).
Tan, M. et al. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell 146, 1016–1028 (2011).
Green, D. R. & Kroemer, G. Cytoplasmic functions of the tumor suppressor p53. Nature 458, 1127 (2009).
Huangyang, P. & Simon, M. C. Hidden features: exploring the non-canonical functions of metabolic enzymes. Dis. Model. Mech. 11, dmm033365 (2018).
Loh, K. H. et al. Proteomic analysis of unbounded cellular compartments: synaptic clefts. Cell 166, 1295–1307 (2016). This study is the first to use proximity labelling to study the synaptic-cleft proteome in neuron cultures and to report proteins specific to excitatory versus inhibitory synapses.
Zuchero, Y. J. Y. et al. Discovery of novel blood–brain barrier targets to enhance brain uptake of therapeutic antibodies. Neuron 89, 70–82 (2016).
Itzhak, D. N., Tyanova, S., Cox, J. & Borner, G. H. Global, quantitative and dynamic mapping of protein subcellular localization. eLife 5, e16950 (2016).
Aebersold, R. & Mann, M. Mass-spectrometric exploration of proteome structure and function. Nature 422, 347–355 (2016).
Paulo, J. A., Mancias, J. D. & Gygi, S. P. Proteome-wide protein expression profiling across five pancreatic cell lines. Pancreas 46, 690–698 (2017).
Giansanti, P., Tsiatsiani, L., Low, T. Y. & Heck, A. J. R. Six alternative proteases for mass spectrometry-based proteomics beyond trypsin. Nat. Protoc. 11, 993–1006 (2016).
Huttlin, E. L. et al. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell 143, 1174–1189 (2010).
Sharma, K. et al. Ultradeep human phosphoproteome reveals a distinct regulatory nature of Tyr and Ser/Thr-based signaling. Cell Rep. 8, 1583–1594 (2014).
Choudhary, C. et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325, 834–840 (2009).
Schölz, C. et al. Acetylation site specificities of lysine deacetylase inhibitors in human cells. Nat. Biotechnol. 33, 415–423 (2015).
Kim, W. et al. Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol. Cell 44, 325–340 (2011).
Rose, C. M. et al. Highly multiplexed quantitative mass spectrometry analysis of ubiquitylomes. Cell Syst. 3, 395–403 (2016).
Hornbeck, P. V. et al. PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res. 43, D512–D520 (2015).
Weerapana, E. et al. Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature 468, 790–795 (2010).
Qu, Z. et al. Proteomic quantification and site-mapping of S-nitrosylated proteins using isobaric iodoTMT reagents. J. Proteome Res. 13, 3200–3211 (2014).
Erickson, B. K. et al. A strategy to combine sample multiplexing with targeted proteomics assays for high-throughput protein signature characterization. Mol. Cell 65, 361–370 (2017).
Huttlin, E. L. et al. Architecture of the human interactome defines protein communities and disease networks. Nature 545, 505–509 (2017).
Huttlin, E. L. et al. The bioplex network: a systematic exploration of the human interactome. Cell 162, 425–440 (2015).
Bakalarski, C. E. & Kirkpatrick, D. S. A biologist’s field guide to multiplexed quantitative proteomics. Mol. Cell. Proteom. 15, 1489–1497 (2016).
Lapek Jr, J. D. et al. Detection of dysregulated protein-association networks by high-throughput proteomics predicts cancer vulnerabilities. Nat. Biotechnol. 35, 983–989 (2017). This study uses high-throughput quantitative proteomics to define predictors of the sensitivity of breast cancer cell lines to a panel of 195 drugs, based on dysregulation of protein–protein interactions.
Klaeger, S. et al. The target landscape of clinical kinase drugs. Science 358, eaan4368 (2017). This study reports a comprehensive list of targets for 243 clinical kinase inhibitors through chemical proteome profiling in four cell lines.
Esbroeck, A. C. M van et al. Activity-based protein profiling reveals off-target proteins of the FAAH inhibitor BIA 10-2474. Science 356, 1084–1087 (2017).
Rhee, H.-W. et al. Proteomic mapping of mitochondria in living cells via spatially restricted enzymatic tagging. Science 339, 1328–1331 (2013).
Roux, K. J., Kim, D. I., Raida, M. & Burke, B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J. Cell Biol. 196, 801–810 (2012).
Dunkley, T. P. J., Watson, R., Griffin, J. L., Dupree, P. & Lilley, K. S. Localization of organelle proteins by isotope tagging (LOPIT). Mol. Cell. Proteom. 3, 1128–1134 (2004).
Orre, L. M. et al. SubCellBarCode: proteome-wide mapping of protein localization and relocalization. Mol. Cell 73, 166–182 (2019). This study applies tandem mass tag (TMT)-based quantification for organelle correlation profiling in order to assess changes in protein localization upon the inhibition of EGFR signalling in lung cancer cells.
Thul, P. J. et al. A subcellular map of the human proteome. Science 356, eaal3321 (2017).
Gygi, S. P., Rochon, Y., Franza, B. R. & Aebersold, R. Correlation between protein and mRNA abundance in yeast. Mol. Cell. Biol. 19, 1720–1730 (1999).
Schwanhäusser, B. et al. Global quantification of mammalian gene expression control. Nature 473, 337–342 (2011).
Pettersson, M. & Crews, C. M. PROteolysis TArgeting chimeras (PROTACs) — past, present and future. Drug Discov. Today Technol. 31, 15–27 (2019).
Kesarwala, A. H., Samrakandi, M. M. & Piwnica-Worms, D. Proteasome inhibition blocks ligand-induced dynamic processing and internalization of epidermal growth factor receptor via altered receptor ubiquitination and phosphorylation. Cancer Res. 69, 976–983 (2009).
Lemmon, M. A., Schlessinger, J. & Ferguson, K. M. The EGFR family: not so prototypical receptor tyrosine kinases. Cold Spring Harb. Perspect. Biol. 6, a020768 (2014).
Francavilla, C. et al. Multilayered proteomics reveals molecular switches dictating ligand-dependent EGFR trafficking. Nat. Struct. Mol. Biol. 23, 608–618 (2016).
Loibl, S. & Gianni, L. HER2-positive breast cancer. Lancet 389, 2415–2429 (2017).
Olsen, J. V. et al. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 127, 635–648 (2006).
Bose, R. et al. Phosphoproteomic analysis of Her2/neu signaling and inhibition. Proc. Natl Acad. Sci. USA 103, 9773–9778 (2006).
Rexer, B. N. et al. Phosphoproteomic mass spectrometry profiling links Src family kinases to escape from HER2 tyrosine kinase inhibition. Oncogene 30, 4163–4174 (2011).
Choudhary, C. et al. Mislocalized activation of oncogenic RTKs switches downstream signaling outcomes. Mol. Cell 36, 326–339 (2009).
Guo, A. et al. Signaling networks assembled by oncogenic EGFR and c-Met. Proc. Natl Acad. Sci. USA 105, 692–697 (2008).
Pan, C., Olsen, J. V., Daub, H. & Mann, M. Global effects of kinase inhibitors on signaling networks revealed by quantitative phosphoproteomics. Mol. Cell. Proteom. 8, 2796–2808 (2009).
Zhuang, G. et al. Phosphoproteomic analysis implicates the mTORC2–FoxO1 axis in VEGF signaling and feedback activation of receptor tyrosine kinases. Sci. Signal. 6, ra25 (2013).
Yamaguchi, H., Chang, S.-S., Hsu, J. L. & Hung, M.-C. Signaling cross-talk in the resistance to HER family receptor targeted therapy. Oncogene 33, 1073–1081 (2014).
Boyer, A. P., Collier, T. S., Vidavsky, I. & Bose, R. Quantitative proteomics with siRNA screening identifies novel mechanisms of trastuzumab resistance in HER2 amplified breast cancers. Mol. Cell. Proteom. 12, 180–193 (2013).
Liu, L. et al. Novel mechanism of lapatinib resistance in HER2-positive breast tumor cells: activation of AXL. Cancer Res. 69, 6871–6878 (2009).
Liu, W. et al. Quantitative proteomics profiling reveals activation of mTOR pathway in trastuzumab resistance. Oncotarget 8, 45793–45806 (2017).
Li, J. et al. A chemical and phosphoproteomic characterization of dasatinib action in lung cancer. Nat. Chem. Biol. 6, 291–299 (2010).
Duncan, J. S. et al. Dynamic reprogramming of the kinome in response to targeted MEK inhibition in triple negative breast cancer. Cell 149, 307–321 (2012). This study uses multiplexed kinase inhibitor beads and mass spectrometry (MIB-MS) to define kinome reprogramming in triple-negative breast cancer cell lines treated with the MEK inhibitor AZD6244.
Stuhlmiller, T. J. et al. Inhibition of lapatinib-induced kinome reprogramming in ERBB2-positive breast cancer by targeting BET family bromodomains. Cell Rep. 11, 390–404 (2015).
McNeill, R. S. et al. Combination therapy with potent PI3K and MAPK inhibitors overcomes adaptive kinome resistance to single agents in preclinical models of glioblastoma. Neuro-Oncol. 19, 1469–1480 (2017).
Ciceri, P. et al. Dual kinase-bromodomain inhibitors for rationally designed polypharmacology. Nat. Chem. Biol. 10, 305–312 (2014).
Lappano, R. & Maggiolini, M. G protein-coupled receptors: novel targets for drug discovery in cancer. Nat. Rev. Drug. Discov. 10, 47–60 (2011).
Thompson, G. L., Kelly, E., Christopoulos, A. & Canals, M. Novel GPCR paradigms at the μ-opioid receptor. Br. J. Pharmacol. 172, 287–296 (2015).
Gundry, J., Glenn, R., Alagesan, P. & Rajagopal, S. A practical guide to approaching biased agonism at G protein coupled receptors. Front. Neurosci. 11, 17 (2017).
Tsvetanova, N. G. et al. G protein–coupled receptor endocytosis confers uniformity in responses to chemically distinct ligands. Mol. Pharmacol. 91, 145–156 (2017).
Williams, G. R. et al. Exploring G protein-coupled receptor signaling networks using SILAC-based phosphoproteomics. Methods 92, 36–50 (2016).
Ong, S.-E. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteom. 1, 376–386 (2002).
Christensen, G. L. et al. Quantitative phosphoproteomics dissection of seven-transmembrane receptor signaling using full and biased agonists. Mol. Cell. Proteom. 9, 1540–1553 (2010).
Bantscheff, M. et al. Chemoproteomics profiling of HDAC inhibitors reveals selective targeting of HDAC complexes. Nat. Biotechnol. 29, 255–265 (2011).
Molina, D. M. et al. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science 341, 84–87 (2013). This seminal study describes the development and application of cellular thermal shift assay (CETSA) for evaluating target engagement by a drug in intact cells.
Franken, H. et al. Thermal proteome profiling for unbiased identification of direct and indirect drug targets using multiplexed quantitative mass spectrometry. Nat. Protoc. 10, 1567–1593 (2015).
Savitski, M. M. et al. Tracking cancer drugs in living cells by thermal profiling of the proteome. Science 346, 1255784 (2014). This study builds on the CETSA assay by incorporating TMT and quantitative mass spectrometry for proteome-wide analysis of on- and off-target drug effects.
Kerbrat, A. et al. Acute neurologic disorder from an inhibitor of fatty acid amide hydrolase. N. Engl. J. Med. 375, 1717–1725 (2016).
Arastu-Kapur, S. et al. Nonproteasomal targets of the proteasome inhibitors bortezomib and carfilzomib: a link to clinical adverse events. Clin. Cancer Res. 17, 2734–2743 (2011).
Sumi, N. J., Kuenzi, B. M., Knezevic, C. E., Rix, L. L. R. & Rix, U. Chemoproteomics reveals novel protein and lipid kinase targets of clinical CDK4/6 inhibitors in lung cancer. ACS Chem. Biol. 10, 2680–2686 (2015).
Muslin, A. J., Tanner, J. W., Allen, P. M. & Shaw, A. S. Interaction of 14–3–3 with signaling proteins is mediated by the recognition of phosphoserine. Cell 84, 889–897 (1996).
Ramakrishnan, G. et al. AKT and 14-3-3 regulate Notch4 nuclear localization. Sci. Rep. 5, 8782 (2015).
Kanai, F. et al. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 19, 6778–6791 (2000).
Wang, A. H. et al. Regulation of histone deacetylase 4 by binding of 14-3-3 proteins. Mol. Cell Biol. 20, 6904–6912 (2000).
Mezzadra, R. et al. Identification of CMTM6 and CMTM4 as PD-L1 protein regulators. Nature 549, 106–110 (2017).
Burr, M. L. et al. CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity. Nature 549, 101–105 (2017).
Jahan, A. S. et al. Usp12 stabilizes the T-cell receptor complex at the cell surface during signaling. Proc. Natl Acad. Sci. USA 113, E705–E714 (2016).
Kim, J. M. & Chen, D. S. Immune escape to PD-L1/PD-1 blockade: seven steps to success (or failure). Ann. Oncol. 27, 1492–1504 (2016).
Carvalho, A. S., Molina, H. & Matthiesen, R. New insights into functional regulation in MS-based drug profiling. Sci. Rep. 6, srep18826 (2016).
Foster, L. J. et al. A mammalian organelle map by protein correlation profiling. Cell 125, 187–199 (2006).
Geladaki, A. et al. Combining LOPIT with differential ultracentrifugation for high-resolution spatial proteomics. Nat. Commun. 10, 331 (2019).
Uezu, A. et al. Identification of an elaborate complex mediating postsynaptic inhibition. Science 353, 1123–1129 (2016).
Tao, C.-L. et al. Differentiation and characterization of excitatory and inhibitory synapses by cryo-electron tomography and correlative microscopy. J. Neurosci. 38, 1493–1510 (2018).
Kalkat, M. et al. MYC protein interactome profiling reveals functionally distinct regions that cooperate to drive tumorigenesis. Mol. Cell 72, 836–848.e7 (2018).
Adhikari, H. & Counter, C. M. Interrogating the protein interactomes of RAS isoforms identifies PIP5K1A as a KRAS-specific vulnerability. Nat. Commun. 9, 3646 (2018).
Reitsma, J. M. et al. Composition and regulation of the cellular repertoire of SCF ubiquitin ligases. Cell 171, 1326–1339 (2017).
Tackett, A. J. et al. I-DIRT, a general method for distinguishing between specific and nonspecific protein interactions. J. Proteome Res. 4, 1752–1756 (2005).
Joshi, P. et al. The functional interactome landscape of the human histone deacetylase family. Mol. Syst. Biol. 9, 672 (2013).
Phelan, J. D. et al. A multiprotein supercomplex controlling oncogenic signaling in lymphoma. Nature 560, 387–391 (2018). This study uses SILAC-based quantification to characterize a multiprotein subcomplex regulating BCR signalling in ibrutinib-responsive diffuse large B cell lymphoma.
Couzens, A. et al. Protein interaction network of the mammalian hippo pathway reveals mechanisms of kinase-phosphatase interactions. Sci. Signal. 6, rs15 (2013).
Kim, B. R. et al. Identification of the SOX2 interactome by BioID reveals EP300 as a mediator of SOX2-dependent squamous differentiation and lung squamous cell carcinoma growth. Mol. Cell. Proteom. 16, 1864–1888 (2017).
De Munter, S. et al. Split-BioID: a proximity biotinylation assay for dimerization-dependent protein interactions. FEBS Lett. 591, 415–424 (2017).
Schopp, I. M. et al. Split-BioID a conditional proteomics approach to monitor the composition of spatiotemporally defined protein complexes. Nat. Commun. 8, 15690 (2017).
Titeca, K. et al. Analyzing trapped protein complexes by Virotrap and SFINX. Nat. Protoc. 12, 881–898 (2017).
Eyckerman, S. et al. Trapping mammalian protein complexes in viral particles. Nat. Commun. 7, 11416 (2016). This study describes a novel approach for identifying membrane–protein interactions and small-molecule binders by trapping them within viral-like particles (Virotrap).
Steklov, M. et al. Mutations in LZTR1 drive human disease by dysregulating RAS ubiquitination. Science 362, 1177–1182 (2018).
Winter, G. E. et al. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science 348, 1376–1381 (2015).
Bondeson, D. P. et al. Catalytic in vivo protein knockdown by small-molecule PROTACs. Nat. Chem. Biol. 11, 611–617 (2015).
Imami, K. et al. Temporal profiling of lapatinib-suppressed phosphorylation signals in EGFR/HER2 pathways. Mol. Cell. Proteom. 11, 1741–1757 (2012).
Kirkpatrick, D. S. et al. Phosphoproteomic characterization of DNA damage response in melanoma cells following MEK/PI3K dual inhibition. Proc. Natl Acad. Sci. USA 110, 19426–19431 (2013).
Wu, Y. L. et al. Dual inhibition of PI3K/AKT and MEK/ERK pathways induces synergistic antitumor effects in diffuse intrinsic pontine glioma cells. Transl Oncol. 10, 221–228 (2017).
Piunti, A. et al. Therapeutic targeting of polycomb and BET bromodomain proteins in diffuse intrinsic pontine gliomas. Nat. Med. 23, 493–500 (2017).
Mohammad, F. et al. EZH2 is a potential therapeutic target for H3K27M-mutant pediatric gliomas. Nat. Med. 23, 483–492 (2017).
Sacco, F. et al. Glucose-regulated and drug-perturbed phosphoproteome reveals molecular mechanisms controlling insulin secretion. Nat. Commun. 7, 13250 (2016).
Giddey, A. D. et al. A temporal proteome dynamics study reveals the molecular basis of induced phenotypic resistance in mycobacterium smegmatis at sub-lethal rifampicin concentrations. Sci. Rep. 73, 43858 (2017).
Burslem, G. M. et al. The advantages of targeted protein degradation over inhibition: an RTK case study. Cell Chem. Biol. 25, 67–77.e3 (2018).
Sakamoto, K. M. et al. Development of protacs to target cancer-promoting proteins for ubiquitination and degradation. Mol. Cell. Proteom. 2, 1350–1358 (2003).
Paek, J. et al. Multidimensional tracking of GPCR signaling via peroxidase-catalyzed proximity labeling. Cell 169, 338–349.e11 (2017).
Lobingier, B. T. et al. An approach to spatiotemporally resolve protein interaction networks in living cells. Cell 169, 350–360 (2017).
Ordureau, A. et al. Dynamics of PARKIN-dependent mitochondrial ubiquitylation in induced neurons and model systems revealed by digital snapshot proteomics. Mol. Cell 70, 211–227 (2018).
Chen, W. W., Freinkman, E., Wang, T., Birsoy, K. & Sabatini, D. M. Absolute quantification of matrix metabolites reveals the dynamics of mitochondrial metabolism. Cell 166, 1324–1337 (2016).
Weekes, M. P. et al. Quantitative temporal viromics: an approach to investigate host–pathogen interaction. Cell 157, 1460–1472 (2014).
Beltran, P. M., Mathias, R. A. & Cristea, I. M. A portrait of the human organelle proteome in space and time during cytomegalovirus infection. Cell Syst. 3, 361–373 (2016).
Li, J. et al. Spatiotemporal profile of postsynaptic interactomes integrates components of complex brain disorders. Nat. Neurosci. 20, 1150–1161 (2017).
Devaux, S. et al. Proteomic analysis of the spatio-temporal based molecular kinetics of acute spinal cord injury identifies a time- and segment-specific window for effective tissue repair. Mol. Cell. Proteom. 15, 2641–2670 (2016).
Liu, J. J. et al. In vivo brain GPCR signaling elucidated by phosphoproteomics. Science 360, eaao4927 (2018).
Brown, H. A., Thomas, P. G. & Lindsley, C. W. Targeting phospholipase D in cancer, infection and neurodegenerative disorders. Nat. Rev. Drug Discov. 16, 351–367 (2017).
Liu, Q. et al. A proximity-tagging system to identify membrane protein–protein interactions. Nat. Methods 15, 715–722 (2018).
Tian, R. et al. Combinatorial proteomic analysis of intercellular signaling applied to the CD28 T-cell costimulatory receptor. Proc. Natl Acad. Sci. USA 112, E1594–E1603 (2015).
Tape, C. J. et al. Oncogenic KRAS regulates tumor cell signaling via stromal reciprocation. Cell 165, 910–920 (2016).
Naba, A., Clauser, K. R., Lamar, J. M., Carr, S. A. & Hynes, R. O. Extracellular matrix signatures of human mammary carcinoma identify novel metastasis promoters. eLife 3, e01308 (2014).
Wang, X. et al. Breast tumors educate the proteome of stromal tissue in an individualized but coordinated manner. Sci. Signal. 10, eaam8065 (2017).
Bamberger, C. et al. Deducing the presence of proteins and proteoforms in quantitative proteomics. Nat. Commun. 9, 2320 (2018).
Thompson, A. et al. Tandem mass tags: a novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal. Chem. 75, 1895–1904 (2003).
Kirkpatrick, D. S., Gerber, S. A. & Gygi, S. P. The absolute quantification strategy: a general procedure for the quantification of proteins and post-translational modifications. Methods 35, 265–273 (2005).
Gillet, L. C. et al. Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: a new concept for consistent and accurate proteome analysis. Mol. Cell. Proteom. 11, O111.016717 (2012).
Zheng, Y. et al. Temporal regulation of EGF signalling networks by the scaffold protein Shc1. Nature 499, 166–171 (2013).
Reddy, R. J. et al. Early signaling dynamics of the epidermal growth factor receptor. Proc. Natl Acad. Sci. USA 113, 3114–3119 (2016).
Dürnberger, G. et al. Global analysis of muscle-specific kinase signaling by quantitative phosphoproteomics. Mol. Cell. Proteom. 13, 1993–2003 (2014).
Bryson, B. D. & White, F. M. Quantitative profiling of lysine acetylation reveals dynamic crosstalk between receptor tyrosine kinases and lysine acetylation. PLOS ONE 10, e0126242 (2015).
Akimov, V., G. Rigbolt, K. T., M. Nielsen, M. & Blagoev, B. Characterization of ubiquitination dependent dynamics in growth factor receptor signaling by quantitative proteomics. Mol. Biosyst. 7, 3223–3233 (2011).
Nelson, D. E. et al. Oscillations in NF-κB signaling control the dynamics of gene expression. Science 306, 704–708 (2004).
Tian, B., Nowak, D. E. & Brasier, A. R. A TNF-induced gene expression program under oscillatory NF-κB control. BMC Genomics 6, 137 (2005).
Acknowledgements
The authors thank members of the MPL group for their input and support.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
H.G.B. and D.S.K. are employees of Genentech Inc., a wholly owned subsidiary of Roche. D.S.K. is a shareholder in Roche.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Spectral counting
-
Quantitative analysis based on comparing the total number of spectra identified per protein in a mass spectrometry analysis.
- Multiplexed isobaric tagging
-
Mass spectrometry (MS)-based quantification approach, in which labelled peptides from multiple samples are mixed together and analysed by mass spectrometer. Upon peptide fragmentation, reporter ions of different masses are released from the isobaric tags, and their relative intensities are measured within individual MS2/MS3 scan events.
- Chemoproteomics
-
Proteomics approaches that use chemical probes to elucidate small-molecule–protein interactions in the proteome.
- Proximity labelling
-
Proteomics approaches that use genetically engineered enzymes for the labelling and enrichment of proteins within subcellular regions and protein communities.
- Phosphoproteomics
-
Proteomics approaches used for the characterization of proteins post-translationally modified with a phosphate group by kinase enzymes.
- Thermal-shift assay
-
A method used to quantify changes in protein stability under thermal denaturation in response to different treatments or conditions, such as binding of a small molecule.
- Synaptic cleft
-
Space or gap at the synapse between a neuron and an effector cell (such as a neuron or a muscle cell).
- Postsynaptic density
-
Protein-dense compartment at the postsynaptic membrane of a synapse between two neurons.
- Xenobiotics
-
Substances that are foreign to the biological system.
- BioID
-
A mass spectrometry-based method for studying the associations of a protein of interest fused to a biotin ligase that labels other proteins in its close proximity.
- Single-shot mass spectrometry
-
A mass spectrometry experimental setup wherein single sample injections are performed, as opposed to multiple injections of fractionated peptide mixtures.
Rights and permissions
About this article
Cite this article
Budayeva, H.G., Kirkpatrick, D.S. Monitoring protein communities and their responses to therapeutics. Nat Rev Drug Discov 19, 414–426 (2020). https://doi.org/10.1038/s41573-020-0063-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41573-020-0063-y
This article is cited by
-
Noninvasive proteomic biomarkers for alcohol-related liver disease
Nature Medicine (2022)
-
Spatially resolved phosphoproteomics reveals fibroblast growth factor receptor recycling-driven regulation of autophagy and survival
Nature Communications (2022)
-
Patient-level proteomic network prediction by explainable artificial intelligence
npj Precision Oncology (2022)
-
The emerging role of mass spectrometry-based proteomics in drug discovery
Nature Reviews Drug Discovery (2022)
-
Interrogating biological systems using visible-light-powered catalysis
Nature Reviews Chemistry (2021)