Abstract
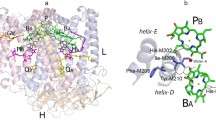
In photosynthetic reaction centers (RCs) of purple bacteria, conserved histidine residues [His L173 and His M202 in Rhodobacter (Rba.) sphaeroides] are known to serve as fifth axial ligands to the central Mg atom of the bacteriochlorophyll (BChl) molecules (PA and PB, respectively) that constitute the homodimer (BChl/BChl) primary electron donor P. In a number of previous studies, it has been found that replacing these residues with leucine, which cannot serve as a ligand to the Mg ion of BChl, leads to the assembly of heterodimer RCs with P represented by the BChl/BPheo pair. Here, we show that a homodimer P is assembled in Rba. sphaeroides RCs if the mutation H(M202)L is combined with the mutation of isoleucine to histidine at position M206 located in the immediate vicinity of PB. The resulting mutant H(M202)L/I(M206)H RCs are characterized using pigment analysis, redox titration, and a number of spectroscopic methods. It is shown that, compared to wild-type RCs, the double mutation causes significant changes in the absorption spectrum of the P homodimer and the electronic structure of the radical cation P+, but has only minor effect on the pigment composition, the P/P+ midpoint potential, and the initial electron-transfer reaction. The results are discussed in terms of the nature of the axial ligand to the Mg of PB in mutant H(M202)L/I(M206)H RCs and the possibility of His M202 participation in the previously proposed through-bond route for electron transfer from the excited state P* to the monomeric BChl BA in wild-type RCs.






Similar content being viewed by others
Abbreviations
- RC:
-
Reaction center
- Wt:
-
Wild type
- BChl:
-
Bacteriochlorophyll
- BPheo:
-
Bacteriopheophytin
- P:
-
Dimer of BChls in the RC
- PA and PB :
-
BChls constituting P
- BA and BB :
-
Monomeric BChls in the active and inactive cofactors branch, respectively
- HA and HB :
-
BPheos in the active and inactive cofactors branch, respectively
- QA :
-
Primary quinone acceptor
- QB :
-
Secondary quinone acceptor
- Rba. :
-
Rhodobacter
- FTIR:
-
Fourier transform infrared
- EPR:
-
Electron paramagnetic resonance
References
Blankenship RE (2014) Molecular mechanisms of photosynthesis. Wiley Blackwell, Chichester
Breton J, Nabedryk E, Parson WW (1992) A new infrared electronic transition of the oxidized primary electron donor in bacterial reaction centers: a way to assess resonance interactions between the bacteriochlorophylls. Biochemistry 31:7503–7510. https://doi.org/10.1021/bi00148a010
Budil DE, Kolaczkowski SV, Norris JR (1987) The temperature dependence of electron back-transfer from the primary radical pair of bacterial photosynthesis. In: Biggins G (ed) Progress in photosynthesis research, vol 1. Martinus Nijhoff Publishers, Dordrecht, pp 25–28
Bylina EJ, Youvan DC (1988) Directed mutations affecting spectroscopic and electron transfer properties of the primary donor in the photosynthetic reaction center. Proc Natl Acad Sci USA 85:7226–7230. https://doi.org/10.1073/pnas.85.19.7226
Camara-Artigas A, Magee C, Goetsch A, Allen JP (2002) The structure of the heterodimer reaction center from Rhodobacter sphaeroides at 2.55 Å resolution. Photosynth Res 74:87–93. https://doi.org/10.1023/A:1020882402389
Chidsey CED, Kirmaier C, Holten D, Boxer SG (1984) Magnetic field dependence of radical-pair decay kinetics and molecular triplet quantum yield in quinone-depleted reaction centers. Biochim Biophys Acta 766:424–437. https://doi.org/10.1016/0005-2728(84)90258-5
DeLano WL (2002) The PyMOL molecular graphics system. https://www.pymol.org
DiMagno TJ, Laible PD, Reddy NR, Small GJ, Norris JR, Schiffer M, Hanson DK (1998) Protein–chromophore interactions: spectral shifts report the consequences of mutations in the bacterial photosynthetic reaction center. Spectrochim Acta A 54:1247–1267. https://doi.org/10.1016/S1386-1425(98)00074-2
Eisenmayer TJ, Lasave JA, Monti A, de Groot HJM, Buda F (2013) Proton displacements coupled to primary electron transfer in the Rhodobacter sphaeroides reaction center. J Phys Chem B 117:11162–11168. https://doi.org/10.1021/jp401195t
Ermler U, Fritzsch G, Buchanan SK, Michel H (1994) Structure of the photosynthetic reaction centre from Rhodobacter sphaeroides at 2.65 Å resolution: cofactors and protein–cofactor interactions. Structure 2:925–936. https://doi.org/10.1016/s0969-2126(94)00094-8
Fufina TY, Vasilieva LG, Gabdulkhakov AG, Shuvalov VA (2015) The L(M196)H mutation in Rhodobacter sphaeroides reaction center results in new electrostatic interactions. Photosynth Res 125:23–29. https://doi.org/10.1007/s11120-014-0062-0
Gibasiewicz K, Białek R, Pajzderska M, Karolczak J, Burdzinski G, Jones MR, Brettel K (2016) Weak temperature dependence of P+HA− recombination in mutant Rhodobacter sphaeroides reaction centers. Photosynth Res 128:243–258. https://doi.org/10.1007/s11120-016-0239-9
Goldsmith JO, King B, Boxer SG (1996) Mg coordination by amino acid side chains is not required for assembly and function of the special pair in bacterial photosynthetic reaction centers. Biochemistry 35:2421–2428. https://doi.org/10.1021/bi9523365
Gudowska-Nowak E, Newton MD, Fajer J (1990) Conformational and environmental effects on bacteriochlorophyll optical spectra: correlations of calculated spectra with structural results. J Phys Chem 94:5795–5801. https://doi.org/10.1021/j100378a036
Heller BA, Holten D, Kirmaier C (1995) Characterization of bacterial reaction centers having mutations of aromatic residues in the binding site of the bacteriopheophytin intermediary electron carrier. Biochemistry 34:5294–5302. https://doi.org/10.1021/bi00015a045
Huber M, Lous EJ, Isaacson RA, Feher G, Gaul D, Schenck CC (1990) EPR and ENDOR studies of the oxidized donor in reaction centers of Rhodobacter sphaeroides strain R-26 and two heterodimer mutants in which histidine M202 or Ll73 was replaced by leucine. In: Michel-Beyerle ME (ed) Reaction centers of photosynthetic bacteria. Springer, Berlin, pp 219–228
Johnson ET, Müh F, Nabedryk E, Williams JC, Allen JP, Lubitz W, Breton J, Parson WW (2002) Electronic and vibronic coupling of the special pair of bacteriochlorophylls in photosynthetic reaction centers from wild-type and mutant strains of Rhodobacter sphaeroides. J Phys Chem B 106:11859–11869. https://doi.org/10.1021/jp021024q
Johnson ET, Nagarajan V, Zazubovich V, Riley K, Small GJ, Parson WW (2003) Effects of ionizable residues on the absorption spectrum and initial electron-transfer kinetics in the photosynthetic reaction center of Rhodobacter sphaeroides. Biochemistry 42:13673–13683. https://doi.org/10.1021/bi035366d
Jones MR, Visschers RW, van Grondelle R, Hunter CN (1992) Construction and characterization of a mutant of Rhodobacter sphaeroides with the reaction center as the sole pigment–protein complex. Biochemistry 31:4458–4465. https://doi.org/10.1021/bi00133a011
Khatypov RA, Vasilieva LG, Fufina TY, Bolgarina TI, Shuvalov VA (2005) Substitution of isoleucine L177 by histidine affects the pigment composition and properties of the reaction center of the purple bacterium Rhodobacter sphaeroides. Biochemistry (Mosc) 70:1256–1261. https://doi.org/10.1007/s10541-005-0256-3
Kirmaier C, Holten D, Bylina EJ, Youvan DC (1988) Electron transfer in a genetically modified bacterial reaction center containing a heterodimer. Proc Natl Acad Sci USA 85:7562–7566. https://doi.org/10.1073/pnas.85.20.7562
Kirmaier C, Gaul D, DeBey R, Holten D, Schenck CC (1991) Charge separation in a reaction center incorporating bacteriochlorophyll for photoactive bacteriopheophytin. Science 251:922–927. https://doi.org/10.1126/science.2000491
Kirmaier C, Laporte L, Schenck CC, Holten D (1995) The nature and dynamics of the charge-separated intermediate in reaction centers in which bacteriochlorophyll replaces the photoactive bacteriopheophytin 2 The rates and yields of charge separation and recombination. J Phys Chem 99:8910–8917. https://doi.org/10.1021/j100021a068
Lin X, Murchison HA, Nagarajan V, Parson WW, Allen JP, Williams JC (1994) Specific alteration of the oxidation potential of the electron donor in reaction centers from Rhodobacter sphaeroides. Proc Natl Acad Sci USA 91:10265–10269. https://doi.org/10.1073/pnas.91.22.10265
Malferrari M, Turina P, Francia F, Mezzetti A, Leibl W, Venturoli G (2015) Dehydration affects the electronic structure of the primary electron donor in bacterial photosynthetic reaction centers: evidence from visible–NIR and light-induced difference FTIR spectroscopy. Photochem Photobiol Sci 14:238–251. https://doi.org/10.1039/c4pp00245h
McDowell LM, Gaul D, Kirmaier C, Holten D, Schenck CC (1991) Investigation into the source of electron transfer asymmetry in bacterial reaction centers. Biochemistry 30:8315–8322. https://doi.org/10.1021/bi00098a006
Moss DA, Leonhard M, Bauscher M, Mäntele W (1991) Electrochemical redox titration of cofactors in the reaction center from Rhodobacter sphaeroides. FEBS Lett 283:33–36. https://doi.org/10.1016/0014-5793(91)80547-G
Müh F, Rautter J, Lubitz W (1996) Effects of zwitterionic detergents on the primary donor of bacterial reaction centers. Ber Bunsenges Phys Chem 100:1974–1977. https://doi.org/10.1002/bbpc.19961001208
Müh F, Rautter J, Lubitz W (1997) Two distinct conformations of the primary electron donor in reaction centers from Rhodobacter sphaeroides revealed by ENDOR/TRIPLE-spectroscopy. Biochemistry 36:4155–4162. https://doi.org/10.1021/bi962859s
Nabedryk E, Robles JSJ, Goldman E, Youvan DC, Breton J (1992) Probing the primary donor environment in the histidine M200-leucine and histidine L173-leucine heterodimer mutants of Rhodobacter capsulatus by light-induced Fourier transform infrared difference spectroscopy. Biochemistry 31:10852–10858. https://doi.org/10.1021/bi00159a028
Nabedryk E, Schulz C, Müh F, Lubitz W, Breton J (2000) Heterodimeric versus homodimeric structure of the primary electron donor in Rhodobacter sphaeroides reaction centers genetically modified at position M202. Photochem Photobiol 71:582–588. https://doi.org/10.1562/0031-8655(2000)071%3C0582:hvhsot%3E2.0.co;2
Naberdyk E, Allen JP, Taguchi AKW, Williams JC, Woodbury NW, Breton J (1993) Fourier transform infrared study of the primary electron donor in chromatophores of Rhodobacter sphaeroides with reaction centers genetically modified at residues M160 and L131. Biochemistry 32:13879–13885. https://doi.org/10.1021/bi00213a017
Ogrodnik A, Volk M, Letterer R, Feick R, Michel-Beyerle ME (1988) Determination of free energies in reaction centers of Rb. sphaeroides. Biochim Biophys Acta 936:361–371. https://doi.org/10.1016/0005-2728(88)90012-6
Parson WW (2008) Functional patterns of reaction centers in anoxygenic photosynthetic bacteria. In: Renger G (ed) Primary processes of photosynthesis, Part 2 principles and apparatus. RSC Publishing, Cambridge, pp 57–109. https://doi.org/10.1039/9781847558169-00057
Parson WW, Warshel A (1987) Spectroscopic properties of photosynthetic reaction centers. 2. Application of the theory to Rhodopseudomonas viridis. J Am Chem Soc 109:6152–6163. https://doi.org/10.1021/ja00254a040
Ponomarenko NS, Li L, Marino AR, Tereshko V, Ostafin A, Popova JA, Bylina EJ, Ismagilov RF, Norris JRJ (2009) Structural and spectropotentiometric analysis of Blastochloris viridis heterodimer mutant reaction center. Biochim Biophys Acta Biomembr 1788:1827–1831. https://doi.org/10.1016/j.bbamem.2009.06.006
Potter JA, Fyfe PK, Frolov D, Wakeham MC, van Grondelle R, Robert B, Jones MR (2005) Strong effects of an individual water molecule on the rate of light-driven charge separation in the Rhodobacter sphaeroides reaction center. J Biol Chem 280:27155–27164. https://doi.org/10.1074/jbc.M501961200
Rautter J, Lendzian F, Schulz C, Fetsch A, Kuhn M, Lin X, Williams JC, Allen JP, Lubitz W (1995) ENDOR studies of the primary donor cation radical in mutant reaction centers of Rhodobacter sphaeroides with altered hydrogen-bond interactions. Biochemistry 34:8130–8143. https://doi.org/10.1021/bi00025a020
Reimers JR, Hush NS (2003) Modeling the bacterial photosynthetic reaction center. VII. Full simulation of the intervalence hole-transfer absorption spectrum of the special-pair radical cation. J Chem Phys 119:3262–3277. https://doi.org/10.1063/1.1589742
Shkuropatov AYa, Shuvalov VA (1993) Electron transfer in pheophytin a-modified reaction centers from Rhodobacter sphaeroides (R-26). FEBS Lett 322:168–172. https://doi.org/10.1016/0014-5793(93)81561-d
Snellenburg JJ, Laptenok SP, Seger R, Mullen KM, van Stokkum IHM (2012) Glotaran: a Java-based graphical user interface for the R package TIMP. J Stat Soft 49:1–22. https://doi.org/10.18637/jss.v049.i03
Spiedel D, Roszak AW, McKendrick K, McAuley KE, Fyfe PK, Nabedryk E, Breton J, Robert B, Cogdell RJ, Isaacs NW, Jones MR (2002) Tuning of the optical and electrochemical properties of the primary donor bacteriochlorophylls in the reaction centre from Rhodobacter sphaeroides: spectroscopy and structure. Biochim Biophys Acta 1554:75–93. https://doi.org/10.1016/s0005-2728(02)00215-3
Sun C, Carey A-M, Gao B-R, Wraight CA, Woodbury NW, Lin S (2016) Ultrafast electron transfer kinetics in the LM dimer of bacterial photosynthetic reaction center from Rhodobacter sphaeroides. J Phys Chem B 120:5395–5404. https://doi.org/10.1021/acs.jpcb.6b05082
Thompson MA, Zerner MC, Fajer J (1991) A theoretical examination of the electronic structure and excited states of the bacteriochlorophyll b dimer from Rhodopseudomonas viridis. J Phys Chem 95:5693–5700. https://doi.org/10.1021/j100167a058
van Stokkum IHM, Larsen DS, van Grondelle R (2004) Global and target analysis of time-resolved spectra. Biochim Biophys Acta 1657:82–104. https://doi.org/10.1016/j.bbabio.2004.04.011
Vasilieva LG, Fufina TY, Gabdulkhakov AG, Leonova MM, Khatypov RA, Shuvalov VA (2012) The site-directed mutation I(L177)H in Rhodobacter sphaeroides reaction center affects coordination of PA and BB bacteriochlorophylls. Biochim Biophys Acta 1817:1407–1417. https://doi.org/10.1016/j.bbabio.2012.02.008
Wang S, Lin S, Lin X, Woodbury NW, Allen JP (1994) Comparative study of reaction centers from purple photosynthetic bacteria: isolation and optical spectroscopy. Photosynth Res 42:203–215. https://doi.org/10.1007/BF00018263
Williams JC, Alden RG, Murchison HA, Peloquin JM, Woodbury NW, Allen JP (1992) Effect of mutations near the bacteriochlorophylls in reaction centers from Rhodobacter sphaeroides. Biochemistry 31:11029–11037. https://doi.org/10.1021/bi00160a012
Woodbury NW, Allen JP (1995) The pathway, kinetics and thermodynamics of electron transfer in wild type and mutant reaction centers of purple nonsulfur bacteria. In: Blankenship RE, Madigan MT, Bauer CE (eds) Anoxygenic photosynthetic bacteria. Kluwer Academic Publishers, Dordrecht, pp 527–557. https://doi.org/10.1007/0-306-47954-0_24
Yakovlev AG, Shkuropatov AYa, Shuvalov VA (2002) Nuclear wave packet motion between P* and P+BA− potential surfaces with a subsequent electron transfer to HA in bacterial reaction centers at 90 K. Electron transfer pathway. Biochemistry 41:14019–14027. https://doi.org/10.1021/bi020250n
Yakovlev AG, Jones MR, Potter JA, Fyfe PK, Vasilieva LG, Shkuropatov AYa, Shuvalov VA (2005) Primary charge separation between P* and BA: electron-transfer pathways in native and mutant GM203L bacterial reaction centers. Chem Phys 319:297–307. https://doi.org/10.1016/j.chemphys.2005.08.018
Zabelin AA, Fufina TY, Vasilieva LG, Shkuropatova VA, Zvereva MG, Shkuropatov AY, Shuvalov VA (2009) Mutant reaction centers of Rhodobacter sphaeroides I(L177)H with strongly bound bacteriochlorophyll a: structural properties and pigment–protein interactions. Biochemistry (Mosc) 74:68–74. https://doi.org/10.1134/S0006297909010106
Zabelin AA, Fufina TYu, Khristin AM, Khatypov RA, Shkuropatova VA, Shuvalov VA, Vasilieva LG, Shkuropatov AYa (2019a) Effect of leucine M196 substitution by histidine on electronic structure of the primary electron donor and electron transfer in reaction centers from Rhodobacter sphaeroides. Biochemistry (Mosc) 84:520–528. https://doi.org/10.1134/S0006297919050067
Zabelin AA, Shkuropatova VA, Shuvalov VA, Shkuropatov AYa (2019b) Spectral and photochemical properties of Rhodobacter sphaeroides R-26 reaction center films in vacuum. Biochemistry (Mosc) 84:1107–1115. https://doi.org/10.1134/S000629791909013X
Acknowledgements
This work was performed under State Task No. AAAA-A17030110140-5 and partially financially supported by the Russian Foundation for Basic Research (Grant No. 17-00-00207 KOMFI).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by AMK, AAZ, and TYuF. The first draft of the manuscript was written by AYaS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. AMK, AAZ, and TYuF are equally contributed to the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khristin, A.M., Zabelin, A.A., Fufina, T.Y. et al. Mutation H(M202)L does not lead to the formation of a heterodimer of the primary electron donor in reaction centers of Rhodobacter sphaeroides when combined with mutation I(M206)H. Photosynth Res 146, 109–121 (2020). https://doi.org/10.1007/s11120-020-00728-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-020-00728-9