Abstract
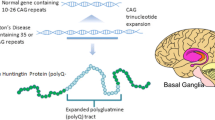
Huntington’s disease is a dominantly inherited neurodegenerative disease caused by an unstable expanded trinucleotide repeat at the short end of the fourth chromosome. Central nervous system pathology begins in the striatum, eventually affecting the entire brain and occurs consequent to multiple intracellular derangements. The proximate cause is a mutant protein with an elongated polyglutamine tract. Pharmacological approaches targeting multiple domains of intracellular functions have universally been disappointing. However, recent developments in gene therapy, including antisense oligonucleotides, small interfering RNAs, and gene editing are bringing new hope to the Huntington’s community. This review discusses the promises and challenges of these new potential treatments.

Similar content being viewed by others
References
Rawlins MD, Wexler NS, Wexler AR, Tabrizi SJ, Douglas I, Evans SJ, et al. The prevalence of Huntington’s disease. Neuroepidemiology. 2016;46(2):144–53.
Paulsen JS, Miller AC, Hayes T, Shaw E. Cognitive and behavioral changes in Huntington disease before diagnosis. Handb Clin Neurol. 2017;144:69–91.
McColgan P, Tabrizi SJ. Huntington’s disease: a clinical review. Eur J Neurol. 2018;25(1):24–34.
Cronin T, Rosser A, Massey T. Clinical presentation and features of juvenile-onset Huntington’s disease: a systematic review. J Huntingtons Dis. 2019;8(2):171–9.
Landles C, Bates GP. Huntingtin and the molecular pathogenesis of Huntington’s disease. Fourth in molecular medicine review series. EMBO Rep. 2004;5(10):958–63.
Wexler NS, Lorimer J, Porter J, Gomez F, Moskowitz C, Shackell E, et al. Venezuelan kindreds reveal that genetic and environmental factors modulate Huntington’s disease age of onset. Proc Natl Acad Sci USA. 2004;101(10):3498–503.
Cattaneo E, Zuccato C, Tartari M. Normal huntingtin function: an alternative approach to Huntington’s disease. Nat Rev Neurosci. 2005;6(12):919–30.
Caron NS, Dorsey ER, Hayden MR. Therapeutic approaches to Huntington disease: from the bench to the clinic. Nat Rev Drug Discov. 2018;17(10):729–50.
Ross CA, Tabrizi SJ. Huntington’s disease: from molecular pathogenesis to clinical treatment. Lancet Neurol. 2011;10(1):83–98.
Lakra P, Aditi K, Agrawal N. Peripheral expression of mutant huntingtin is a critical determinant of weight loss and metabolic disturbances in Huntington’s disease. Sci Rep. 2019;9(1):10127.
Travessa AM, Rodrigues FB, Mestre TA, Ferreira JJ. Fifteen years of clinical trials in Huntington’s disease: a very low clinical drug development success rate. J Huntingtons Dis. 2017;6(2):157–63.
Lee JM, Ramos EM, Lee JH, Gillis T, Mysore JS, Hayden MR, et al. CAG repeat expansion in Huntington disease determines age at onset in a fully dominant fashion. Neurology. 2012;78(10):690–5.
Buren C, Wang L, Smith-Dijak A, Raymond LA. Region-specific pro-survival signaling and global neuronal protection by wild-type Huntingtin. J Huntingtons Dis. 2014;3(4):365–76.
Leavitt BR, Guttman JA, Hodgson JG, Kimel GH, Singaraja R, Vogl AW, et al. Wild-type huntingtin reduces the cellular toxicity of mutant huntingtin in vivo. Am J Hum Genet. 2001;68(2):313–24.
Reilly CE. Wild-type huntingtin up-regulates BDNF transcription in Huntington’s disease. J Neurol. 2001;248(10):920–2.
Reiner A, Dragatsis I, Zeitlin S, Goldowitz D. Wild-type huntingtin plays a role in brain development and neuronal survival. Mol Neurobiol. 2003;28(3):259–76.
Rigamonti D, Bauer JH, De-Fraja C, Conti L, Sipione S, Sciorati C, et al. Wild-type huntingtin protects from apoptosis upstream of caspase-3. J Neurosci. 2000;20(10):3705–13.
Strehlow AN, Li JZ, Myers RM. Wild-type huntingtin participates in protein trafficking between the Golgi and the extracellular space. Hum Mol Genet. 2007;16(4):391–409.
Boudreau RL, McBride JL, Martins I, Shen S, Xing Y, Carter BJ, et al. Nonallele-specific silencing of mutant and wild-type huntingtin demonstrates therapeutic efficacy in Huntington’s disease mice. Mol Ther. 2009;17(6):1053–63.
Grondin R, Kaytor MD, Ai Y, Nelson PT, Thakker DR, Heisel J, et al. Six-month partial suppression of Huntingtin is well tolerated in the adult rhesus striatum. Brain. 2012;135(Pt 4):1197–209.
McBride JL, Pitzer MR, Boudreau RL, Dufour B, Hobbs T, Ojeda SR, et al. Preclinical safety of RNAi-mediated HTT suppression in the rhesus macaque as a potential therapy for Huntington’s disease. Mol Ther. 2011;19(12):2152–62.
Stiles DK, Zhang Z, Ge P, Nelson B, Grondin R, Ai Y, et al. Widespread suppression of huntingtin with convection-enhanced delivery of siRNA. Exp Neurol. 2012;233(1):463–71.
Wild EJ, Boggio R, Langbehn D, Robertson N, Haider S, Miller JR, et al. Quantification of mutant huntingtin protein in cerebrospinal fluid from Huntington’s disease patients. J Clin Invest. 2015;125(5):1979–86.
Ostergaard ME, Southwell AL, Kordasiewicz H, Watt AT, Skotte NH, Doty CN, et al. Rational design of antisense oligonucleotides targeting single nucleotide polymorphisms for potent and allele selective suppression of mutant Huntingtin in the CNS. Nucleic Acids Res. 2013;41(21):9634–50.
Carroll JB, Warby SC, Southwell AL, Doty CN, Greenlee S, Skotte N, et al. Potent and selective antisense oligonucleotides targeting single-nucleotide polymorphisms in the Huntington disease gene/allele-specific silencing of mutant huntingtin. Mol Ther. 2011;19(12):2178–85.
Lombardi MS, Jaspers L, Spronkmans C, Gellera C, Taroni F, Di Maria E, et al. A majority of Huntington’s disease patients may be treatable by individualized allele-specific RNA interference. Exp Neurol. 2009;217(2):312–9.
Pfister EL, Kennington L, Straubhaar J, Wagh S, Liu W, DiFiglia M, et al. Five siRNAs targeting three SNPs may provide therapy for three-quarters of Huntington’s disease patients. Curr Biol. 2009;19(9):774–8.
Kay C, Collins JA, Caron NS, Agostinho LA, Findlay-Black H, Casal L, et al. A comprehensive haplotype targeting strategy for allele-specific HTT suppression in Huntington disease. Am J Hum Genet. 2019;105(6):1112–25.
Dufour BD, Smith CA, Clark RL, Walker TR, McBride JL. Intrajugular vein delivery of AAV9-RNAi prevents neuropathological changes and weight loss in Huntington’s disease mice. Mol Ther. 2014;22(4):797–810.
Harper SQ, Staber PD, He X, Eliason SL, Martins IH, Mao Q, et al. RNA interference improves motor and neuropathological abnormalities in a Huntington’s disease mouse model. Proc Natl Acad Sci USA. 2005;102(16):5820–5.
Keeler AM, Sapp E, Chase K, Sottosanti E, Danielson E, Pfister E, et al. Cellular analysis of silencing the Huntington’s disease gene using AAV9 mediated delivery of artificial micro RNA into the striatum of Q140/Q140 mice. J Huntingtons Dis. 2016;5(3):239–48.
Kordasiewicz HB, Stanek LM, Wancewicz EV, Mazur C, McAlonis MM, Pytel KA, et al. Sustained therapeutic reversal of Huntington’s disease by transient repression of huntingtin synthesis. Neuron. 2012;74(6):1031–44.
Pfister EL, DiNardo N, Mondo E, Borel F, Conroy F, Fraser C, et al. Artificial miRNAs reduce human mutant Huntingtin throughout the striatum in a transgenic sheep model of Huntington’s disease. Hum Gene Ther. 2018;29(6):663–73.
Rue L, Banez-Coronel M, Creus-Muncunill J, Giralt A, Alcala-Vida R, Mentxaka G, et al. Targeting CAG repeat RNAs reduces Huntington’s disease phenotype independently of huntingtin levels. J Clin Invest. 2016;126(11):4319–30.
Stanek LM, Sardi SP, Mastis B, Richards AR, Treleaven CM, Taksir T, et al. Silencing mutant huntingtin by adeno-associated virus-mediated RNA interference ameliorates disease manifestations in the YAC128 mouse model of Huntington’s disease. Hum Gene Ther. 2014;25(5):461–74.
Zamecnik PC, Stephenson ML. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc Natl Acad Sci USA. 1978;75(1):280–4.
Rossor AM, Reilly MM, Sleigh JN. Antisense oligonucleotides and other genetic therapies made simple. Pract Neurol. 2018;18(2):126–31.
Rinaldi C, Wood MJA. Antisense oligonucleotides: the next frontier for treatment of neurological disorders. Nat Rev Neurol. 2018;14(1):9–21.
Wild EJ, Tabrizi SJ. Therapies targeting DNA and RNA in Huntington’s disease. Lancet Neurol. 2017;16(10):837–47.
Geary RS, Norris D, Yu R, Bennett CF. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv Drug Deliv Rev. 2015;29(87):46–51.
Randeree L, Eslick GD. Eteplirsen for paediatric patients with Duchenne muscular dystrophy: a pooled-analysis. J Clin Neurosci. 2018;49:1–6.
Goyal N, Narayanaswami P. Making sense of antisense oligonucleotides: a narrative review. Muscle Nerve. 2018;57(3):356–70.
Tabrizi SJ, Leavitt BR, Landwehrmeyer GB, Wild EJ, Saft C, Barker RA, et al. Targeting Huntingtin expression in patients with Huntington’s disease. N Engl J Med. 2019;380(24):2307–16.
Rodrigues FB, Wild EJ. Huntington’s disease clinical trials corner: February 2018. J Huntingtons Dis. 2018;7(1):89–98.
Wang SY, Chen W, Xu W, Li JQ, Hou XH, Ou YN, et al. Neurofilament light chain in cerebrospinal fluid and blood as a biomarker for neurodegenerative diseases: a systematic review and meta-analysis. J Alzheimers Dis. 2019;72(4):1353–61.
Datson NA, Gonzalez-Barriga A, Kourkouta E, Weij R, van de Giessen J, Mulders S, et al. The expanded CAG repeat in the huntingtin gene as target for therapeutic RNA modulation throughout the HD mouse brain. PLoS One. 2017;12(2):e0171127.
Hammond SM, Hazell G, Shabanpoor F, Saleh AF, Bowerman M, Sleigh JN, et al. Systemic peptide-mediated oligonucleotide therapy improves long-term survival in spinal muscular atrophy. Proc Natl Acad Sci USA. 2016;113(39):10962–7.
Aguiar S, van der Gaag B, Cortese FAB. RNAi mechanisms in Huntington’s disease therapy: siRNA versus shRNA. Transl Neurodegener. 2017;6:30.
Dana H, Chalbatani GM, Mahmoodzadeh H, Karimloo R, Rezaiean O, Moradzadeh A, et al. Molecular mechanisms and biological functions of siRNA. Int J Biomed Sci. 2017;13(2):48–57.
Rodriguez-Lebron E, Denovan-Wright EM, Nash K, Lewin AS, Mandel RJ. Intrastriatal rAAV-mediated delivery of anti-huntingtin shRNAs induces partial reversal of disease progression in R6/1 Huntington’s disease transgenic mice. Mol Ther. 2005;12(4):618–33.
Boudreau RL, Martins I, Davidson BL. Artificial microRNAs as siRNA shuttles: improved safety as compared to shRNAs in vitro and in vivo. Mol Ther. 2009;17(1):169–75.
Miniarikova J, Zanella I, Huseinovic A, van der Zon T, Hanemaaijer E, Martier R, et al. Design, characterization, and lead selection of therapeutic miRNAs targeting Huntingtin for development of gene therapy for Huntington’s disease. Mol Ther Nucleic Acids. 2016;22(5):e297.
Drouet V, Perrin V, Hassig R, Dufour N, Auregan G, Alves S, et al. Sustained effects of nonallele-specific Huntingtin silencing. Ann Neurol. 2009;65(3):276–85.
Franich NR, Fitzsimons HL, Fong DM, Klugmann M, During MJ, Young D. AAV vector-mediated RNAi of mutant huntingtin expression is neuroprotective in a novel genetic rat model of Huntington’s disease. Mol Ther. 2008;16(5):947–56.
Huang BJ, Yin H, Huang YF, Xu JF, Xiong P, Feng W, et al. Gene therapy using adenoviral vector encoding 4-1BBIg gene significantly prolonged murine cardiac allograft survival. Transpl Immunol. 2006;16(2):88–94.
Machida Y, Okada T, Kurosawa M, Oyama F, Ozawa K, Nukina N. rAAV-mediated shRNA ameliorated neuropathology in Huntington disease model mouse. Biochem Biophys Res Commun. 2006;343(1):190–7.
Alterman JF, Godinho B, Hassler MR, Ferguson CM, Echeverria D, Sapp E, et al. A divalent siRNA chemical scaffold for potent and sustained modulation of gene expression throughout the central nervous system. Nat Biotechnol. 2019;37(8):884–94.
Chaudhary RK, Patel KA, Patel MK, Joshi RH, Roy I. Inhibition of aggregation of mutant Huntingtin by nucleic acid aptamers in vitro and in a yeast model of Huntington’s disease. Mol Ther. 2015;23(12):1912–26.
Shin JW, Kim KH, Chao MJ, Atwal RS, Gillis T, MacDonald ME, et al. Permanent inactivation of Huntington’s disease mutation by personalized allele-specific CRISPR/Cas9. Hum Mol Genet. 2016;25(20):4566–76.
Skogen M, Roth J, Yerkes S, Parekh-Olmedo H, Kmiec E. Short G-rich oligonucleotides as a potential therapeutic for Huntington’s disease. BMC Neurosci. 2006;2(7):65.
Khan E, Biswas S, Mishra SK, Mishra R, Samanta S, Mishra A, et al. Rationally designed small molecules targeting toxic CAG repeat RNA that causes Huntington’s disease (HD) and spinocerebellar ataxia (SCAs). Biochimie. 2019;163:21–32.
Khan E, Tawani A, Mishra SK, Verma AK, Upadhyay A, Kumar M, et al. Myricetin reduces toxic level of CAG repeats RNA in Huntington’s disease (HD) and spino cerebellar ataxia (SCAs). ACS Chem Biol. 2018;13(1):180–8.
Agustin-Pavon C, Mielcarek M, Garriga-Canut M, Isalan M. Deimmunization for gene therapy: host matching of synthetic zinc finger constructs enables long-term mutant Huntingtin repression in mice. Mol Neurodegener. 2016;11(1):64.
Garriga-Canut M, Agustin-Pavon C, Herrmann F, Sanchez A, Dierssen M, Fillat C, et al. Synthetic zinc finger repressors reduce mutant huntingtin expression in the brain of R6/2 mice. Proc Natl Acad Sci USA. 2012;109(45):E3136–45.
Zeitler B, Froelich S, Marlen K, Shivak DA, Yu Q, Li D, et al. Allele-selective transcriptional repression of mutant HTT for the treatment of Huntington’s disease. Nat Med. 2019;25(7):1131–42.
Lino CA, Harper JC, Carney JP, Timlin JA. Delivering CRISPR: a review of the challenges and approaches. Drug Deliv. 2018;25(1):1234–57.
Fink KD, Deng P, Gutierrez J, Anderson JS, Torrest A, Komarla A, et al. Allele-specific reduction of the mutant Huntingtin allele using transcription activator-like effectors in human Huntington’s disease fibroblasts. Cell Transplant. 2016;25(4):677–86.
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–21.
Richardson CD, Ray GJ, Bray NL, Corn JE. Non-homologous DNA increases gene disruption efficiency by altering DNA repair outcomes. Nat Commun. 2016;17(7):12463.
Savic N, Schwank G. Advances in therapeutic CRISPR/Cas9 genome editing. Transl Res. 2016;168:15–21.
Yang S, Chang R, Yang H, Zhao T, Hong Y, Kong HE, et al. CRISPR/Cas9-mediated gene editing ameliorates neurotoxicity in mouse model of Huntington’s disease. J Clin Invest. 2017;127(7):2719–24.
Merienne N, Vachey G, de Longprez L, Meunier C, Zimmer V, Perriard G, et al. The self-inactivating KamiCas9 system for the editing of CNS disease genes. Cell Rep. 2017;20(12):2980–91.
Kosicki M, Tomberg K, Bradley A. Repair of double-strand breaks induced by CRISPR-Cas9 leads to large deletions and complex rearrangements. Nat Biotechnol. 2018;36(8):765–71.
Ross CA, Aylward EH, Wild EJ, Langbehn DR, Long JD, Warner JH, et al. Huntington disease: natural history, biomarkers and prospects for therapeutics. Nat Rev Neurol. 2014;10(4):204–16.
Bonelli RM, Hodl AK, Hofmann P, Kapfhammer HP. Neuroprotection in Huntington’s disease: a 2-year study on minocycline. Int Clin Psychopharmacol. 2004;19(6):337–42.
Ferreira JJ, Rosser A, Craufurd D, Squitieri F, Mallard N, Landwehrmeyer B. Ethyl-eicosapentaenoic acid treatment in Huntington’s disease: a placebo-controlled clinical trial. Mov Disord. 2015;30(10):1426–9.
Huntington Study G. A randomized, placebo-controlled trial of coenzyme Q10 and remacemide in Huntington’s disease. Neurology. 2001;57(3):397–404.
Huntington Study Group DI. A futility study of minocycline in Huntington’s disease. Mov Disord. 2010;25(13):2219–24.
Rosas HD, Doros G, Gevorkian S, Malarick K, Reuter M, Coutu JP, et al. PRECREST: a phase II prevention and biomarker trial of creatine in at-risk Huntington disease. Neurology. 2014;82(10):850–7.
Shoulson I, Odoroff C, Oakes D, Behr J, Goldblatt D, Caine E, et al. A controlled clinical trial of baclofen as protective therapy in early Huntington’s disease. Ann Neurol. 1989;25(3):252–9.
Sussmuth SD, Haider S, Landwehrmeyer GB, Farmer R, Frost C, Tripepi G, et al. An exploratory double-blind, randomized clinical trial with selisistat, a SirT1 inhibitor, in patients with Huntington’s disease. Br J Clin Pharmacol. 2015;79(3):465–76.
Thomas M, Ashizawa T, Jankovic J. Minocycline in Huntington’s disease: a pilot study. Mov Disord. 2004;19(6):692–5.
Stout JC, Queller S, Baker KN, Cowlishaw S, Sampaio C, Fitzer-Attas C, et al. HD-CAB: a cognitive assessment battery for clinical trials in Huntington’s disease 1,2,3. Mov Disord. 2014;29(10):1281–8.
Paulsen JS, Lourens S, Kieburtz K, Zhang Y. Sample enrichment for clinical trials to show delay of onset in huntington disease. Mov Disord. 2019;34(2):274–80.
Aylward EH, Nopoulos PC, Ross CA, Langbehn DR, Pierson RK, Mills JA, et al. Longitudinal change in regional brain volumes in prodromal Huntington disease. J Neurol Neurosurg Psychiatry. 2011;82(4):405–10.
Feigin A, Ghilardi MF, Huang C, Ma Y, Carbon M, Guttman M, et al. Preclinical Huntington’s disease: compensatory brain responses during learning. Ann Neurol. 2006;59(1):53–9.
Feigin A, Tang C, Ma Y, Mattis P, Zgaljardic D, Guttman M, et al. Thalamic metabolism and symptom onset in preclinical Huntington’s disease. Brain. 2007;130(Pt 11):2858–67.
Tang CC, Feigin A, Ma Y, Habeck C, Paulsen JS, Leenders KL, et al. Metabolic network as a progression biomarker of premanifest Huntington’s disease. J Clin Invest. 2013;123(9):4076–88.
Cotter K, Siskind CE, Sha SJ, Hanson-Kahn AK. Positive attitudes and therapeutic misconception around hypothetical clinical trial participation in the Huntington’s disease community. J Huntingtons Dis. 2019;8(4):421–30.
Osmand AP, Bichell TJ, Bowman AB, Bates GP. Embryonic mutant Huntingtin agregate formation in mouse models of Huntington’s disease. J Huntingtons Dis. 2016;5(4):343–6.
Nopoulos PC, Aylward EH, Ross CA, Mills JA, Langbehn DR, Johnson HJ, et al. Smaller intracranial volume in prodromal Huntington’s disease: evidence for abnormal neurodevelopment. Brain. 2011;134(Pt 1):137–42.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this article.
Conflict of interest
Kathleen M. Shannon has no conflicts that are directly relevant to the content of this article.
Rights and permissions
About this article
Cite this article
Shannon, K.M. Recent Advances in the Treatment of Huntington’s Disease: Targeting DNA and RNA. CNS Drugs 34, 219–228 (2020). https://doi.org/10.1007/s40263-019-00695-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-019-00695-3