Abstract
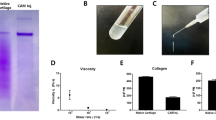
Osteoarthritis (OA) is a general joint disease. Cartilage damage is associated with a decrease in the density of chondrocytes. Mesenchymal stem cells (MSCs) differentiate into adipocytes, osteocytes and chondrocytes, and are an excellent source of cell therapy. Cartilage-derived extracellular matrix (ECM) promotes chondrogenesis of MSCs. However, the role of MSCs stimulated by ECM is not well known in OA. The purpose of this study is to determine the role of specific factors generated by the application of ECM and umbilical cord blood-derived mesenchymal stem cells (UCB-MSCs) in managing OA symptoms. Cartilage acellular matrix (CAM), which is a cartilage-derived ECM, was used to promote the chondrogenesis of UCB-MSCs. Induced MSCs were analyzed using chondrogenic markers (aggrecan, collagen type 2, and SOX9) and bone morphogenic protein 6 (BMP6). BMP6 is known to be involved in early chondrogenesis of MSCs. As a result, treatment with CAM significantly increased the expression of chondrogenic markers and BMP6 in UCB-MSCs. Treatment with recombinant human BMP6 also dramatically increased the levels of chondrogenic markers in UCB-MSCs. In addition, UCB-MSCs and CAM were used to evaluate OA symptom improvement in a rabbit articular cruciate ligament transection (ACLT) model. Application of UCB-MSCs and CAM enhanced not only the structure and synthesis of proteoglycan and collagen type 2 but also anti-inflammatory effects in both rabbit joint and synovial fluid. Moreover, the detection of human cells and involvement of BMP6 were confirmed in rabbit cartilage tissues. This study indicates that therapeutic potential of UCB-MSCs with CAM is mediated via BMP6 in OA.








Similar content being viewed by others
References
Verbrugge, L. M. (1995). Women, men, and osteoarthritis. Arthritis Care and Research, 8(4), 212–220. https://doi.org/10.1002/art.1790080404.
Crepaldi, G., & Punzi, L. (2003). Aging and osteoarthritis. Aging Clinical and Experimental Research, 15(5), 355–358. https://doi.org/10.1007/bf03327355.
Bijlsma, J. W., Berenbaum, F., & Lafeber, F. P. (2011). Osteoarthritis: An update with relevance for clinical practice. Lancet, 377(9783), 2115–2126. https://doi.org/10.1016/S0140-6736(11)60243-2.
Loeser, R. F., Goldring, S. R., Scanzello, C. R., & Goldring, M. B. (2012). Osteoarthritis: A disease of the joint as an organ. Arthritis and Rheumatism, 64(6), 1697–1707. https://doi.org/10.1002/art.34453.
Maldonado, M., & Nam, J. (2013). The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. BioMed Research International, 2013(284873), 1–10. https://doi.org/10.1155/2013/284873.
Akkiraju, H., & Nohe, A. (2015). Role of chondrocytes in cartilage formation, progression of osteoarthritis and cartilage regeneration. Journal of Developmental Biology, 3(4), 177–192. https://doi.org/10.3390/jdb3040177.
Li, Y., Wei, X., Zhou, J., & Wei, L. (2013). The age-related changes in cartilage and osteoarthritis. BioMed Research International, 2013(916530). https://doi.org/10.1155/2013/916530.
McAlindon, T. E., Bannuru, R. R., Sullivan, M. C., Arden, N. K., Berenbaum, F., Bierma-Zeinstra, S. M., et al. (2014). OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis and Cartilage, 22(3), 363–388. https://doi.org/10.1016/j.joca.2014.01.003.
Gupta, P. K., Das, A. K., Chullikana, A., & Majumdar, A. S. (2012). Mesenchymal stem cells for cartilage repair in osteoarthritis. Stem Cell Research and Therapy, 3(4), 25. https://doi.org/10.1186/scrt116.
Zuk, P. A., Zhu, M., Ashjian, P., De Ugarte, D. A., Huang, J. I., Mizuno, H., et al. (2002). Human adipose tissue is a source of multipotent stem cells. Molecular Biology of the Cell, 13(12), 4279–4295. https://doi.org/10.1091/mbc.e02-02-0105.
Colter, D. C., Class, R., DiGirolamo, C. M., & Prockop, D. J. (2000). Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proceedings of the National Academy of Sciences of the United States of America, 97(7), 3213–3218. https://doi.org/10.1073/pnas.070034097.
Miranda, J. P., Filipe, E., Fernandes, A. S., Almeida, J. M., Martins, J. P., De la Fuente, A., et al. (2015). The human umbilical cord tissue-derived MSC population UCX((R)) promotes early Motogenic effects on keratinocytes and fibroblasts and G-CSF-mediated mobilization of BM-MSCs when transplanted in vivo. Cell Transplantation, 24(5), 865–877. https://doi.org/10.3727/096368913X676231.
Parolini, O., Alviano, F., Bagnara, G. P., Bilic, G., Buhring, H. J., Evangelista, M., . . . & Strom, S. C. (2008). Concise review: Isolation and characterization of cells from human term placenta: Outcome of the first international workshop on placenta derived stem cells. Stem Cells, 26(2), 300–311. doi:https://doi.org/10.1634/stemcells.2007-0594.
Lee, B. C., Shin, N., Lee, J. Y., Kang, I., Kim, J. J., Lee, S. E., et al. (2018). MIS416 enhances therapeutic functions of human umbilical cord blood-derived Mesenchymal stem cells against experimental colitis by modulating systemic immune milieu. Frontiers in Immunology, 28(9), 1078. https://doi.org/10.3389/fimmu.2018.01078.
Lee, Y. S., Sah, S. K., Lee, J. H., Seo, K. W., Kang, K. S., & Kim, T. Y. (2017). Human umbilical cord blood-derived mesenchymal stem cells ameliorate psoriasis-like skin inflammation in mice. Biochemistry and Biophysics Reports, 8(9), 281–288. https://doi.org/10.1016/j.bbrep.2016.10.002.
Park, E. H., Lim, H. S., Lee, S., Roh, K., Seo, K. W., Kang, K. S., & Shin, K. (2018). Intravenous infusion of umbilical cord blood-derived Mesenchymal stem cells in rheumatoid arthritis: A phase Ia clinical trial. Stem Cells Translational Medicine, 7(9), 636–642. https://doi.org/10.1002/sctm.18-0031.
Kim, H. S., Yun, J. W., Shin, T. H., Lee, S. H., Lee, B. C., Yu, K. R., et al. (2015). Human umbilical cord blood mesenchymal stem cell-derived PGE2 and TGF-beta1 alleviate atopic dermatitis by reducing mast cell degranulation. Stem Cells, 33(4), 1254–1266. https://doi.org/10.1002/stem.1913.
Sun, B., Jeong, Y. H., Jung, J. W., Seo, K., Lee, Y. S., & Kang, K. S. (2007). Regulation of human umbilical cord blood-derived multi-potent stem cells by autogenic osteoclast-based niche-like structure. Biochemical and Biophysical Research Communications, 357(1), 92–98. https://doi.org/10.1016/j.bbrc.2007.03.072.
Wang, M., Yang, Y., Yang, D., Luo, F., Liang, W., Guo, S., & Xu, J. (2009). The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immunology, 126(2), 220–232. https://doi.org/10.1111/j.1365-2567.2008.02891.x.
Bieback, K., Kern, S., Kluter, H., & Eichler, H. (2004). Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells, 22(4), 625–634. https://doi.org/10.1634/stemcells.22-4-625.
Jeong, S. Y., Kim, D. H., Ha, J., Jin, H. J., Kwon, S. J., Chang, J. W., Choi, S. J., Oh, W., Yang, Y. S., Kim, G., Kim, J. S., Yoon, J. R., Cho, D. H., & Jeon, H. B. (2013). Thrombospondin-2 secreted by human umbilical cord blood-derived mesenchymal stem cells promotes chondrogenic differentiation. Stem Cells, 31(10), 2136–2148. https://doi.org/10.1002/stem.1471.
Park, Y. B., Ha, C. W., Kim, J. A., Han, W. J., Rhim, J. H., Lee, H. J., Kim, K. J., Park, Y. G., & Chung, J. Y. (2017). Single-stage cell-based cartilage repair in a rabbit model: Cell tracking and in vivo chondrogenesis of human umbilical cord blood-derived mesenchymal stem cells and hyaluronic acid hydrogel composite. Osteoarthritis and Cartilage, 25(4), 570–580. https://doi.org/10.1016/j.joca.2016.10.012.
Mifune, Y., Matsumoto, T., Takayama, K., Ota, S., Li, H., Meszaros, L. B., et al. (2013). The effect of platelet-rich plasma on the regenerative therapy of muscle derived stem cells for articular cartilage repair. Osteoarthritis and Cartilage, 21(1), 175–185. https://doi.org/10.1016/j.joca.2012.09.018.
Hermeto, L. C., DeRossi, R., Oliveira, R. J., Pesarini, J. R., Antoniolli-Silva, A. C., Jardim, P. H., Santana, A. E., Deffune, E., Rinaldi, J. C., & Justulin, L. A. (2016). Effects of intra-articular injection of mesenchymal stem cells associated with platelet-rich plasma in a rabbit model of osteoarthritis. Genetics and Molecular Research, 15(3). https://doi.org/10.4238/gmr.15038569.
Yun, S., Ku, S. K., & Kwon, Y. S. (2016). Adipose-derived mesenchymal stem cells and platelet-rich plasma synergistically ameliorate the surgical-induced osteoarthritis in beagle dogs. Journal of Orthopaedic Surgery and Research, 11(9), 1–12. https://doi.org/10.1186/s13018-016-0342-9.
Lv, X., He, J., Zhang, X., Luo, X., He, N., Sun, Z., Xia, H., Liu, V., Zhang, L., Lin, X., Lin, L., Yin, H., Jiang, D., Cao, W., Wang, R., Zhou, G., & Wang, W. (2018). Comparative efficacy of autologous stromal vascular fraction and autologous adipose-derived Mesenchymal stem cells combined with hyaluronic acid for the treatment of sheep osteoarthritis. Cell Transplantation, 27(7), 1111–1125. https://doi.org/10.1177/0963689718773333.
Chiang, E. R., Ma, H. L., Wang, J. P., Liu, C. L., Chen, T. H., & Hung, S. C. (2016). Allogeneic Mesenchymal stem cells in combination with hyaluronic acid for the treatment of osteoarthritis in rabbits. PLoS One, 11(2), e0149835. https://doi.org/10.1371/journal.pone.0149835.
Yin, H., Wang, Y., Sun, Z., Sun, X., Xu, Y., Li, P., et al. (2016). Induction of mesenchymal stem cell chondrogenic differentiation and functional cartilage microtissue formation for in vivo cartilage regeneration by cartilage extracellular matrix-derived particles. Acta Biomaterialia, 33, 96–109. https://doi.org/10.1016/j.actbio.2016.01.024.
Baek, J. H., Kim, K., Yang, S. S., Park, S. H., Song, B. R., Yun, H. W., . . . & Kim, M. S. (2016). Preparation of Extracellular Matrix Developed Using Porcine Articular Cartilage and In Vitro Feasibility Study of Porcine Articular Cartilage as an Anti-Adhesive Film. Materials (Basel), 9(1), pii: E49. doi:10.3390/ma9010049.
Kim, H. J., Lee, S., Yun, H. W., Yin, X. Y., Kimm, S. H., Choi, B. H., et al. (2016). In vivo degradation profile of porcine cartilage-derived extracellular matrix powder scaffolds using a non-invasive fluorescence imaging method. Journal of Biomaterials Science, Polymer Edition, 27(2), 177–190. https://doi.org/10.1080/09205063.2015.1120262.
Benders, K. E., van Weeren, P. R., Badylak, S. F., Saris, D. B., Dhert, W. J., & Malda, J. (2013). Extracellular matrix scaffolds for cartilage and bone regeneration. Trends in Biotechnology, 31(3), 169–176. https://doi.org/10.1016/j.tibtech.2012.12.004.
Bragdon, B., Moseychuk, O., Saldanha, S., King, D., Julian, J., & Nohe, A. (2011). Bone morphogenetic proteins: A critical review. Cellular Signalling, 23(4), 609–620. https://doi.org/10.1016/j.cellsig.2010.10.003.
Sekiya, I., Vuoristo, J. T., Larson, B. L., & Prockop, D. J. (2002). In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proceedings of National Academy of Science of the United States of America, 99(7), 4397–4402. https://doi.org/10.1073/pnas.052716199.
Sekiya, I., Colter, D. C., & Prockop, D. J. (2001). BMP-6 enhances chondrogenesis in a subpopulation of human marrow stromal cells. Biochemedical and Biophysical Research Communications, 284(2), 411–418. https://doi.org/10.1006/bbrc.2001.4898.
Boskey, A. L., Paschalis, E. P., Binderman, I., & Doty, S. B. (2002). BMP-6 accelerates both chondrogenesis and mineral maturation in differentiating chick limb-bud mesenchymal cell cultures. Journal of Cellular Biochemistry, 84(3), 509–519.
Hildner, F., Peterbauer, A., Wolbank, S., Nurnberger, S., Marlovits, S., Redl, H., . . . & Gabriel, C. (2010). FGF-2 abolishes the chondrogenic effect of combined BMP-6 and TGF-beta in human adipose derived stem cells. Journal of Biomedical Materials Research. Part A, 94(3), 978–987. doi:https://doi.org/10.1002/jbm.a.32761.
Diekman, B. O., Estes, B. T., & Guilak, F. (2010). The effects of BMP6 overexpression on adipose stem cell chondrogenesis: Interactions with dexamethasone and exogenous growth factors. Journal of Biomedical Materials Research. Part A, 93(3), 994–1003. doi:https://doi.org/10.1002/jbm.a.32589.
Seo, Y., Yang, S. R., Jee, M. K., Joo, E. K., Roh, K. H., Seo, M. S., Han, T. H., Lee, S. Y., Ryu, P. D., Jung, J. W., Seo, K. W., Kang, S. K., & Kang, K. S. (2011). Human umbilical cord blood-derived mesenchymal stem cells protect against neuronal cell death and ameliorate motor deficits in Niemann pick type C1 mice. Cell Transplantation, 20(7), 1033–1047. https://doi.org/10.3727/096368910X545086.
Kwon, J. S., Yoon, S. M., Shim, S. W., Park, J. H., Min, K. J., Oh, H. J., Kim, J. H., Kim, Y. J., Yoon, J. J., Choi, B. H., & Kim, M. S. (2013). Injectable extracellular matrix hydrogel developed using porcine articular cartilage. International Journal of Pharmaceutics, 454(1), 183–191. https://doi.org/10.1016/j.ijpharm.2013.06.023.
Hamid, A. A., Idrus, R. B., Saim, A. B., Sathappan, S., & Chua, K. H. (2012). Characterization of human adipose-derived stem cells and expression of chondrogenic genes during induction of cartilage differentiation. Clinics (São Paulo, Brazil), 67(2), 99–106. https://doi.org/10.6061/clinics/2012(02)03.
Jin, C. Z., Park, S. R., Choi, B. H., Park, K., & Min, B. H. (2007). In vivo cartilage tissue engineering using a cell-derived extracellular matrix scaffold. Artificial Organs, 31(3), 183–192. https://doi.org/10.1111/j.1525-1594.2007.00363.x.
Badylak, S. F., Freytes, D. O., & Gilbert, T. W. (2009). Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomaterialia, 5(1), 1–13. https://doi.org/10.1016/j.actbio.2008.09.013.
Talakoob, S., Joghataei, M. T., Parivar, K., Bananej, M., & Sanadgol, N. (2015). Capability of cartilage extract to in vitro differentiation of rat Mesenchymal stem cells (MSCs) to chondrocyte lineage. International Journal of Molecular and Cellular Medicine, 4(1), 9–21.
Xue, J. X., Gong, Y. Y., Zhou, G. D., Liu, W., Cao, Y., & Zhang, W. J. (2012). Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells induced by acellular cartilage sheets. Biomaterials, 33(24), 5832–5840. https://doi.org/10.1016/j.biomaterials.2012.04.054.
Park, W. S., Ahn, S. Y., Sung, S. I., Ahn, J. Y., & Chang, Y. S. (2018). Strategies to enhance paracrine potency of transplanted mesenchymal stem cells in intractable neonatal disorders. Pediatric Research, 83(1–2), 214–222. https://doi.org/10.1038/pr.2017.249.
Meretoja, V. V., Dahlin, R. L., Kasper, F. K., & Mikos, A. G. (2012). Enhanced chondrogenesis in co-cultures with articular chondrocytes and mesenchymal stem cells. Biomaterials, 33(27), 6362–6369. https://doi.org/10.1016/j.biomaterials.2012.05.042.
Lee, S. Y., Nakagawa, T., & Reddi, A. H. (2008). Induction of chondrogenesis and expression of superficial zone protein (SZP)/lubricin by mesenchymal progenitors in the infrapatellar fat pad of the knee joint treated with TGF-beta1 and BMP-7. Biochemedical and Biophysical Research Communications, 376(1), 148–153. https://doi.org/10.1016/j.bbrc.2008.08.138.
Yoon, B. S., & Lyons, K. M. (2004). Multiple functions of BMPs in chondrogenesis. Journal of Cellular Biochemistry, 93(1), 93–103. https://doi.org/10.1002/jcb.20211.
Chu, C. R., Szczodry, M., & Bruno, S. (2010). Animal models for cartilage regeneration and repair. Tissue Engineering. Part B. Reviews, 16(1), 105–115. doi:https://doi.org/10.1089/ten.TEB.2009.0452.
Kuyinu, E. L., Narayanan, G., Nair, L. S., & Laurencin, C. T. (2016). Animal models of osteoarthritis: Classification, update, and measurement of outcomes. Journal of Orthopaedic Surgery and Research, 11, 19. https://doi.org/10.1186/s13018-016-0346-5.
van Buul, G. M., Villafuertes, E., Bos, P. K., Waarsing, J. H., Kops, N., Narcisi, R., et al. (2012). Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthritis and Cartilage, 20(10), 1186–1196. https://doi.org/10.1016/j.joca.2012.06.003.
Kehoe, O., Cartwright, A., Askari, A., El Haj, A. J., & Middleton, J. (2014). Intra-articular injection of mesenchymal stem cells leads to reduced inflammation and cartilage damage in murine antigen-induced arthritis. Journal of Translational Medicine, 12, 157. https://doi.org/10.1186/1479-5876-12-157.
Huleihel, L., Hussey, G. S., Naranjo, J. D., Zhang, L., Dziki, J. L., Turner, N. J., Stolz, D. B., & Badylak, S. F. (2016). Matrix-bound nanovesicles within ECM bioscaffolds. Science Advances, 2(6), e1600502. https://doi.org/10.1126/sciadv.1600502.
Zhang, B., Yin, Y., Lai, R. C., Tan, S. S., Choo, A. B., & Lim, S. K. (2014). Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells and Development, 23(11), 1233–1244. https://doi.org/10.1089/scd.2013.0479.
Mokarizadeh, A., Delirezh, N., Morshedi, A., Mosayebi, G., Farshid, A. A., & Mardani, K. (2012). Microvesicles derived from mesenchymal stem cells: Potent organelles for induction of tolerogenic signaling. Immunology Letters, 147(1–2), 47–54. https://doi.org/10.1016/j.imlet.2012.06.001.
Grimsrud, C. D., Romano, P. R., D'Souza, M., Puzas, J. E., Schwarz, E. M., Reynolds, P. R., et al. (2001). BMP signaling stimulates chondrocyte maturation and the expression of Indian hedgehog. Journal of Orthopaedic Research, 19(1), 18–25. https://doi.org/10.1016/S0736-0266(00)00017-6.
Author information
Authors and Affiliations
Contributions
Conception and design of the study, KWS, YBK, KSK; acquisition of data, HJJ, KAY, ESA, TWK, YBS; analysis and interpretation of data, HJJ, SHL, EKC, JCA; drafting the manuscript, HJJ, JCA; All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
This study received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Ethical Approval
This experiment was approved by the Institutional Animal Care and Use Committee (IACUC) of Chungbuk National University Laboratory Animal Research Center and performed in accordance with the Standard Operation Procedures (SOP) of the same facility.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jeon, HJ., Yoon, KA., An, E.S. et al. Therapeutic Effects of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Combined with Cartilage Acellular Matrix Mediated Via Bone Morphogenic Protein 6 in a Rabbit Model of Articular Cruciate Ligament Transection. Stem Cell Rev and Rep 16, 596–611 (2020). https://doi.org/10.1007/s12015-020-09958-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-020-09958-9