Abstract
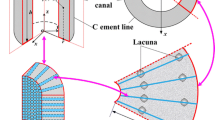
Mechanical loading-induced fluid flow in lacunar–canalicular space (LCS) of bone excites osteocyte cells to release signalling molecules which initiate osteo-activities. Theoretical models considered canaliculi as a uniform and symmetrical space/channel in bone. However, experimental studies reported that canalicular walls are irregular and curvy resulting in inhomogeneous fluid motion which may influence the molecular transport. Therefore, a new mathematical model of LCS with curvy canalicular walls is developed to characterize cantilever bending-induced canalicular flow behaviour in terms of pore-pressure, fluid velocity, and streamlines. The model also analyses the mobility of signalling molecules involved in bone mechanotransduction as a function of loading frequency and permeability of LCS. Inhomogeneous flow is observed at higher loading frequency which amplifies mechanotransduction; nevertheless, it also promotes trapping of signalling molecules. The effects of shape and size of signalling molecules on transport behaviour are also studied. Trivially, signalling molecules larger in size and weight move slower as compared to molecules small in size and weight which validates the findings of the present study. The outcomes will ultimately be useful in designing better biomechanical exercise in combination with pharmaceutical agents to improve the bone health.










Similar content being viewed by others
References
Aboelkassem Y (2012) Novel bioinspired pumping models for microscale flow transport. PhD Thesis, Virginia Tech
Aboelkassem Y (2015) Insect-inspired micropump: flow in a tube with local contractions. Micromachines 6:1143–1156
Aboelkassem Y (2019) Pumping flow model in a microchannel with propagative rhythmic membrane contraction. Phys Fluids 31:051902
Adachi T, Kameo Y, Hojo M (2010) Trabecular bone remodelling simulation considering osteocytic response to fluid-induced shear stress. Philos Trans R Soc Math Phys Eng Sci 368:2669–2682
Akbar N, Tripathi D, Khan Z, Bég OA (2018) Mathematical modelling of pressure-driven micropolar biological flow due to metachronal wave propulsion of beating cilia. Math Biosci 301:121–128
Akhter MP, Cullen DM, Recker RR (2002) Bone adaptation response to sham and bending stimuli in mice. J Clin Densitom 5:207–216
Alexandre C, Vico L (2011) Pathophysiology of bone loss in disuse osteoporosis. Jt Bone Spine 78:572–576
Bacabac RG, Smit TH, Mullender MG et al (2004) Nitric oxide production by bone cells is fluid shear stress rate dependent. Biochem Biophys Res Commun 315:823–829
Bellido T (2014) Osteocyte-driven bone remodeling. Calcif Tissue Int 94:25–34
Bhatti M, Zeeshan A, Ellahi R et al (2019) Effects of coagulation on the two-phase peristaltic pumping of magnetized Prandtl biofluid through an endoscopic annular geometry containing a porous medium. Chin J Phys 58:222–234
Biot MA (1941) General theory of three-dimensional consolidation. J Appl Phys 12:155–164
Biot MA (1955) Theory of elasticity and consolidation for a porous anisotropic solid. J Appl Phys 26:182–185
Bonewald LF (2006) Mechanosensation and transduction in osteocytes. BoneKEy Osteovision 3:7
Burger EH, Klein-Nulend J (1999) Mechanotransduction in bone—role of the lacuno-canalicular network. FASEB J 13:S101–S112
Burger EH, Klein-Nulend J, Cowin SC (1998) Mechanotransduction in bone. Adv Organ Biol 5:123–136
Calbet J, Moysi J, Dorado C, Rodriguez L (1998) Bone mineral content and density in professional tennis players. Calcif Tissue Int 62:491–496
Carriero A, Pereira A, Wilson A et al (2018) Spatial relationship between bone formation and mechanical stimulus within cortical bone: combining 3D fluorochrome mapping and poroelastic finite element modelling. Bone Rep 8:72–80
Ellahi R, Hussain F, Ishtiaq F, Hussain A (2019a) Peristaltic transport of Jeffrey fluid in a rectangular duct through a porous medium under the effect of partial slip: an application to upgrade industrial sieves/filters. Pramana 93:34
Ellahi R, Zeeshan A, Hussain F, Asadollahi A (2019b) Peristaltic blood flow of couple stress fluid suspended with nanoparticles under the influence of chemical reaction and activation energy. Symmetry 11:276
Fan L, Pei S, Lucas L, Wang L (2016) A multiscale 3D finite element analysis of fluid/solute transport in mechanically loaded bone. Bone Res 4:16032. https://doi.org/10.1038/boneres.2016.32
Frost HM (1997) On our age-related bone loss: insights from a new paradigm. J Bone Miner Res 12:1539–1546
Gross TS, Srinivasan S, Liu CC, Clemens TL, Bain SD (2002) Noninvasive loading of the murine tibia: an in vivo model for the study of mechanotransduction. J Bone Miner Res 17(3):493–501
Hambli R, Kourta A (2015) A theory for internal bone remodeling based on interstitial fluid velocity stimulus function. Appl Math Model 39:3525–3534
Hsieh Y-F, Turner CH (2001) Effects of loading frequency on mechanically induced bone formation. J Bone Miner Res 16:918–924
Javed S, Sohail A, Maqbool K et al (2017) The lattice Boltzmann method and computational analysis of bone dynamics-I. Complex Adapt Syst Model 5:12
Jiménez-Lozano J, Sen M, Dunn PF (2009) Particle motion in unsteady two-dimensional peristaltic flow with application to the ureter. Phys Rev E 79:041901
Johnson RW (2016) Handbook of fluid dynamics. CRC Press, Boca Raton
Johnson DL, McAllister TN, Frangos JA (1996) Fluid flow stimulates rapid and continuous release of nitric oxide in osteoblasts. Am J Physiol Endocrinol Metab 271:E205–E208
Kameo Y, Adachi T, Hojo M (2008) Transient response of fluid pressure in a poroelastic material under uniaxial cyclic loading. J Mech Phys Solids 56:1794–1805
Kameo Y, Adachi T, Hojo M (2009) Fluid pressure response in poroelastic materials subjected to cyclic loading. J Mech Phys Solids 57:1815–1827
Kamioka H, Kameo Y, Imai Y et al (2012) Microscale fluid flow analysis in a human osteocyte canaliculus using a realistic high-resolution image-based three-dimensional model. Integr Biol 4:1198. https://doi.org/10.1039/c2ib20092a
Klein-Nulend J, Bakker AD, Bacabac RG et al (2013) Mechanosensation and transduction in osteocytes. Bone 54:182–190
Knothe TM, Niederer P, Knothe U (1998) In vivo tracer transport through the lacunocanalicular system of rat bone in an environment devoid of mechanical loading. Bone 22:107–117
LaMothe JM, Hamilton NH, Zernicke RF (2005) Strain rate influences periosteal adaptation in mature bone. Med Eng Phys 27:277–284
Lau RY, Guo X (2011) A review on current osteoporosis research: with special focus on disuse bone loss. J Osteoporos 2011:1–6. https://doi.org/10.4061/2011/293808
Li M, Brasseur JG (1993) Non-steady peristaltic transport in finite-length tubes. J Fluid Mech 248:129–151
Li W, You L, Schaffler MB, Wang L (2009) The dependency of solute diffusion on molecular weight and shape in intact bone. Bone 45:1017–1023
Meakin LB, Price JS, Lanyon LE (2014) The contribution of experimental in vivo models to understanding the mechanisms of adaptation to mechanical loading in bone. Front Endocrinol 5:154
Mohamad N, Soelaiman I-N, Chin K-Y (2016) A concise review of testosterone and bone health. Clin Interv Aging 11:1317
Montgomery RJ, Sutker BD, Bronk JT et al (1988) Interstitial fluid flow in cortical bone. Microvasc Res 35:295–307
Owan I, Burr DB, Turner CH et al (1997) Mechanotransduction in bone: osteoblasts are more responsive to fluid forces than mechanical strain. Am J Physiol Cell Physiol 273:C810–C815
Palombaro KM (2005) Effects of walking-only interventions on bone mineral density at various skeletal sites: a meta-analysis. J Geriatr Phys Ther 28:102–107
Pereira AF, Shefelbine SJ (2014) The influence of load repetition in bone mechanotransduction using poroelastic finite-element models: the impact of permeability. Biomech Model Mechanobiol 13:215–225
Pereira AF, Javaheri B, Pitsillides A, Shefelbine S (2015) Predicting cortical bone adaptation to axial loading in the mouse tibia. J R Soc Interface 12:20150590
Piekarski K (1977) Transport mechanism operating between blood supply and osteocytes in long bones. Nature 269:80–82
Prasad J, Wiater BP, Nork SE et al (2010) Characterizing gait induced normal strains in a murine tibia cortical bone defect model. J Biomech 43:2765–2770
Price C, Zhou X, Li W, Wang L (2011) Real-time measurement of solute transport within the lacunar–canalicular system of mechanically loaded bone: direct evidence for load-induced fluid flow. J Bone Miner Res 26:277–285
Prideaux M, Findlay DM, Atkins GJ (2016) Osteocytes: the master cells in bone remodelling. Curr Opin Pharmacol 28:24–30
Qin Y-X, Kaplan T, Saldanha A, Rubin C (2003) Fluid pressure gradients, arising from oscillations in intramedullary pressure, is correlated with the formation of bone and inhibition of intracortical porosity. J Biomech 36:1427–1437
Reich KM, Frangos JA (1991) Effect of flow on prostaglandin E2 and inositol trisphosphate levels in osteoblasts. Am J Physiol Cell Physiol 261:C428–C432
Rubin CT, Lanyon LE (1985) Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int 37:411–417
Schaffler MB, Cheung W-Y, Majeska R, Kennedy O (2014) Osteocytes: master orchestrators of bone. Calcif Tissue Int 94:5–24
Shapiro AH, Jaffrin MY, Weinberg SL (1969) Peristaltic pumping with long wavelengths at low Reynolds number. J Fluid Mech 37:799–825
Sheikholeslami M, Ellahi R, Shafee A, Li Z (2019) Numerical investigation for second law analysis of ferrofluid inside a porous semi annulus: an application of entropy generation and exergy loss. Int J Numer Methods Heat Fluid Flow 29:1079–1102
Silva MJ, Brodt MD (2008) Mechanical stimulation of bone formation is normal in the SAMP6 mouse. Calcif Tissue Int 82:489–497
Smit TH, Huyghe JM, Cowin SC (2002) Estimation of the poroelastic parameters of cortical bone. J Biomech 35:829–835
Srinivasan S, Gross T (2000) Canalicular fluid flow induced by bending of a long bone. Med Eng Phys 22:127–133
Srinivasan S, Weimer DA, Agans SC et al (2002) Low-magnitude mechanical loading becomes osteogenic when rest is inserted between each load cycle. J Bone Miner Res 17:1613–1620
Sugiyama T, Meakin LB, Browne WJ et al (2012) Bones’ adaptive response to mechanical loading is essentially linear between the low strains associated with disuse and the high strains associated with the lamellar/woven bone transition. J Bone Miner Res 27:1784–1793
Takabatake S, Ayukawa K, Mori A (1988) Peristaltic pumping in circular cylindrical tubes: a numerical study of fluid transport and its efficiency. J Fluid Mech 193:267–283
Tate MK, Knothe U (2000) An ex vivo model to study transport processes and fluid flow in loaded bone. J Biomech 33:247–254
Tate MK, Steck R, Forwood M, Niederer P (2000) In vivo demonstration of load-induced fluid flow in the rat tibia and its potential implications for processes associated with functional adaptation. J Exp Biol 203:2737–2745
Tiwari AK, Kumar R, Tripathi D, Badhyal S (2018) In silico modeling of bone adaptation to rest-inserted loading: strain energy density versus fluid flow as stimulus. J Theor Biol 446:110–127
Tripathi D (2012) Peristaltic hemodynamic flow of couple-stress fluids through a porous medium with slip effect. Transp Porous Media 92:559–572
Tripathi D (2013) Study of transient peristaltic heat flow through a finite porous channel. Math Comput Model 57:1270–1283
Turner CH, Pavalko FM (1998) Mechanotransduction and functional response of the skeleton to physical stress: the mechanisms and mechanics of bone adaptation. J Orthop Sci 3:346–355
van Tol AF, Roschger A, Repp F et al (2019) Network architecture strongly influences the fluid flow pattern through the lacunocanalicular network in human osteons. Biomech Model Mechanobiol 1–18
Verbruggen SW, Vaughan TJ, McNamara LM (2014) Fluid flow in the osteocyte mechanical environment: a fluid–structure interaction approach. Biomech Model Mechanobiol 13:85–97
Vico L, Collet P, Guignandon A et al (2000) Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet 355:1607–1611
Wang L (2018) Solute transport in the bone lacunar–canalicular system (LCS). Curr Osteoporos Rep 16:32–41
Wang L, Cowin SC, Weinbaum S, Fritton SP (2000) Modeling tracer transport in an osteon under cyclic loading. Ann Biomed Eng 28:1200–1209
Wang L, Wang Y, Han Y et al (2005) In situ measurement of solute transport in the bone lacunar–canalicular system. Proc Natl Acad Sci U S A 102:11911–11916
Wang Y, McNamara LM, Schaffler MB, Weinbaum S (2007) A model for the role of integrins in flow induced mechanotransduction in osteocytes. Proc Natl Acad Sci 104:15941–15946
Wang B, Zhou X, Price C et al (2013) Quantifying load-induced solute transport and solute-matrix interaction within the osteocyte lacunar–canalicular system. J Bone Miner Res 28:1075–1086
Weinbaum S, Cowin S, Zeng Y (1994) A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech 27:339–360
You J, Yellowley C, Donahue H et al (2000) Substrate deformation levels associated with routine physical activity are less stimulatory to bone cells relative to loading-induced oscillatory fluid flow. J Biomech Eng 122:387–393
You L, Temiyasathit S, Lee P et al (2008) Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone 42:172–179
Zhang D, Weinbaum S, Cowin SC (1998) On the calculation of bone pore water pressure due to mechanical loading. Int J Solids Struct 35:4981–4997
Zhang P, Tanaka SM, Jiang H et al (2006) Diaphyseal bone formation in murine tibiae in response to knee loading. J Appl Physiol 100:1452–1459
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, R., Tiwari, A.K., Tripathi, D. et al. Signalling molecule transport analysis in lacunar–canalicular system. Biomech Model Mechanobiol 19, 1879–1896 (2020). https://doi.org/10.1007/s10237-020-01314-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-020-01314-7