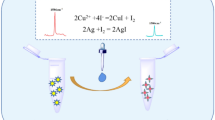
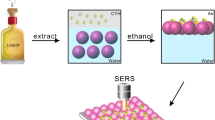
Abstract
Cyanide (C≡N) can lead to blood, cardiovascular system, and nervous system disorders owing to the acute and chronic toxicity; thus, aiming at the group or individual poisoning incidents, it is necessary to develop the sensitive and credible method for rapid on-site detection of poisons cyanide. Surface-enhanced Raman spectroscopy (SERS) with the advantages of providing fingerprint information of target molecules and single-molecules sensitivity has been widely used in on-site analysis; however, the SERS measurements always suffer from the problem of the stability of substrates. Here, the polyvinylpyrrolidone (PVP)-stabilized Au NPs (PVP-Au NPs) have been assembled through the simple, convenient evaporation-induced strategy with the large-scale hotspots substrates. The presence of PVP can not only facilitate the assembly of Au NPs but also prevent the corrosion of CN− towards the Au NPs with the formation of [Au (CN)2]−1, providing high stable and reproducible SERS signals. Moreover, the PVP-Au NPs have been assembled on the Si wafer to fabricate the portable SERS chip for rapid on-site detection of CN− with an RSD of 5.8% and limitation of 100 ppb. Furthermore, by coupling a portable Raman spectrometer, the SERS spectra of CN− spiked into different specimens to simulate the poison samples have been collected and analyzed on SERS chips with the recovery of 89–103% and RSD not higher than 11.3%. Consequently, the fabricated SERS chip with assembled PVP-Au NPs can provide sensitive and credible detection for CN− in different specimens, and then would satisfy the rapid on-site evaluation of CN− in poisoning incidents with the portable Raman spectrometer.

Graphical Abstract





Similar content being viewed by others
References
Chen D, Taylor KP, Hall Q, Kaplan JM. The neuropeptides FLP-2 and PDF-1 act in concert to arouse Caenorhabditis elegans locomotion. Genetics. 2016;204:1151–9.
Lindsay AE, O’hare D. The development of an electrochemical sensor for the determination of cyanide in physiological solutions. Anal Chim Acta. 2006;558:158–63.
Zhang Q, Maddukuri N, Gong M. A direct and rapid method to determine cyanide in urine by capillary electrophoresis. J Chromatogr A. 2015;1414:158–62.
Calafat AM, Stanfill SB. Rapid quantitation of cyanide in whole blood by automated headspace gas chromatography. J Chromatogr B. 2002;772:131–7.
Kang HI, Shin HS. Derivatization method of free cyanide including cyanogen chloride for the sensitive analysis of cyanide in chlorinated drinking water by liquid chromatography-tandem mass spectrometry. Anal Chem. 2015;87:975–81.
Cheng XH, Tang RL, Jia HZ, Feng J, Qin JG, Li Z. New fluorescent and colorimetric probe for cyanide: direct reactivity, high selectivity, and bioimaging application. ACS Appl Mater Interfaces. 2012;4:4387–92.
Jackson R, Oda RP, Bhandari RK, Mahon SB, Brenner M, Rockwood GA, et al. Development of a fluorescence-based sensor for rapid diagnosis of cyanide exposure. Anal Chem. 2014;86:1845–52.
Zhu QX, Cao YB, Cao YY, Chai YF, Lu F. Rapid on-site TLC-SERS detection of four antidiabetes drugs used as adulterants in botanical dietary supplements. Anal Bioanal Chem. 2014;406:1877–84.
Li DA, Lv DY, Zhu QX, Li H, Chen H, Wu MM, et al. Chromatographic separation and detection of contaminants from whole milk powder using a chitosan-modified silver nanoparticles surface-enhanced Raman scattering device. Food Chem. 2017;224:382–9.
Zong C, Xu MX, Xu LJ, Wei T, Ma X, Zheng XS, et al. Surface-enhanced Raman spectroscopy for bioanalysis: reliability and challenges. Chem Rev. 2018;118:4946–80.
Pilo-Pais M, Watson A, Demers S, Labean TH, Finkelstein G. Surface-enhanced Raman scattering plasmonic enhancement using DNA origami-based complex metallic nanostructures. Nano Lett. 2014;14:2099–104.
Dieringer JA, Lettan RB, Scheidt KA, Van Duyne RP. A frequency domain existence proof of single-molecule surface-enhanced Raman spectroscopy. J Am Chem Soc. 2007;129:16249–56.
Wang H, Levin CS, Halas NJ. Nanosphere arrays with controlled sub-10-nm gaps as surface-enhanced Raman spectroscopy substrates. J Am Chem Soc. 2005;127:14992–3.
Peng B, Li GY, Li DH, Dodson S, Zhang Q, Zhang J, et al. Vertically aligned gold nanorod monolayer on arbitrary substrates: self-assembly and femtomolar detection of food contaminants. ACS Nano. 2013;7:5993–6000.
Zhou B, Li X, Tang X, Li P, Yang L, Liu J. Highly selective and repeatable surface-enhanced resonance Raman scattering detection for epinephrine in serum based on interface self-assembled 2D nanoparticles arrays. ACS Appl Mater Interfaces. 2017;9:7772–9.
Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys Sci. 1973;241:20–2.
Lim DK, Jeon KS, Hwang JH, Kim H, Kwon S, Suh YD, et al. Highly uniform and reproducible surface-enhanced Raman scattering from DNA-tailorable nanoparticles with 1-nm interior gap. Nat Nanotechnol. 2011;6:452–60.
Kralchevsky PA, Denkov ND. Capillary forces and structuring in layers of colloid particles. Curr Opin Colloid In. 2001;6:383–401.
Mezzenga R, Ruokolainen J, Hexemer A. On the role of block copolymers in self-assembly of dense colloidal polymeric systems. Langmuir : the ACS journal of surfaces and colloids. 2003;19:8144–7.
Yan Q, Gao L, Sharma V, Chiang YM, Wong CC. Particle and substrate charge effects on colloidal self-assembly in a sessile drop. Langmuir : the ACS journal of surfaces and colloids. 2008;24:11518–22.
Haiss W, Thanh NTK, Aveyard J, Fernig DG. Determination of size and concentration of gold nanoparticles from UV-Vis spectra. Anal Chem. 2007;79:4215–21.
Zhang L, Zhang J, Zheng Z, Liao Y, Xu Y, Li Z, et al. Interaction-transferable graphene-isolated superstable AuCo nanocrystal-enabled direct cyanide capture. Anal Chem. 2019;91:8762–6.
Muehlethaler C, Leona M, Lombardi JR. Review of surface enhanced Raman scattering applications in forensic science. Anal Chem. 2016;88:152–69.
Chen HY, Lin MH, Wang CY, Chang YM, Gwo S. Large-scale hot spot engineering for quantitative SERS at the single-molecule scale. J Am Chem Soc. 2015;137:13698–705.
Ma J, Ohira S, Mishra SK, Puanngam M, Dasgupta PK, Mahon SB, et al. Rapid point of care analyzer for the measurement of cyanide in blood. Anal Chem. 2011;83:4319–24.
Lindsay AE, Greenbaum AR, O’hare D. Analytical techniques for cyanide in blood and published blood cyanide concentrations from healthy subjects and fire victims. Anal Chim Acta. 2004;511:185–95.
Tian Y, Dasgupta PK, Mahon SB, Ma J, Brenner M, Wang JH, et al. A disposable blood cyanide sensor. Anal Chim Acta. 2013;768:129–35.
Gao J, Guo L, Wu J, Feng J, Wang S, Lai F, et al. Simple and sensitive detection of cyanide using pinhole shell-isolated nanoparticle-enhanced Raman spectroscopy. J Raman Spectrosc. 2014;45:619–26.
Funding
The project was supported by the National Natural Science Foundation of China (No. 21365004), Nature Science Research Project of Anhui province (No. 1908085QB65), The Key Research and Development Project of Guangxi (AB18126048), Guangxi Science and Technology Major Project (Gui Ke AA18118013-10), Guangxi University for Nationalities Master Student Innovation Project (gxun-chxps201822).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 483 kb)
Rights and permissions
About this article
Cite this article
Li, P., Li, P., Tan, X. et al. Assembling PVP-Au NPs as portable chip for sensitive detection of cyanide with surface-enhanced Raman spectroscopy. Anal Bioanal Chem 412, 2863–2871 (2020). https://doi.org/10.1007/s00216-020-02517-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02517-8