Abstract
The most effective treatment of Parkinson’s disease is restoring central dopamine levels with levodopa, the metabolic precursor of dopamine. However, due to extensive peripheral metabolism by aromatic l-amino acid decarboxylase and catechol-O-methyltransferase (COMT), only a fraction of the levodopa dose reaches the brain unchanged. Thus, by preventing levodopa metabolism and increasing the availability of levodopa for uptake into the brain, the inhibition of COMT would be beneficial in Parkinson’s disease. Although nitrocatechol COMT inhibitors have been used in the treatment of Parkinson’s disease, efforts have been made to discover non-nitrocatechol inhibitors. In the present study, the 3-hydroxypyridin-4-one scaffold was selected for the design and synthesis of non-nitrocatechol COMT inhibitors since the COMT inhibitory potential of this class has been illustrated. Using COMT obtained from porcine liver, it was shown that a synthetic series of ten 3-hydroxypyridin-4-ones are in vitro inhibitors with IC50 values ranging from 4.55 to 19.8 µM. Although these compounds are not highly potent inhibitors, they may act as leads for the development of non-nitrocatechol COMT inhibitors. Such compounds would be appropriate for the treatment of Parkinson’s disease.
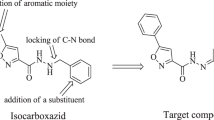
Graphic abstract
3-Hydroxypyridin-4-ones have been synthesised and evaluated as non-nitrocatechol COMT inhibitors. In vitro, the IC50 values ranged from 4.55 to 19.8 μM.

Similar content being viewed by others
Introduction
Parkinson’s disease is a neurodegenerative disorder with bradykinesia, resting tremor and rigidity as the core clinical features. The motor symptoms of Parkinson’s disease are the result of the functional loss of dopamine in the striatum due to the degeneration of the nigrostriatal neuronal pathway [1, 2]. The exact cause of Parkinson’s disease is unknown, and symptoms worsen over time as the disease progresses. Although current pharmacotherapy provides symptomatic relief, no treatment has been proven to slow or halt the progression of the disease [3]. Levodopa, the metabolic precursor of dopamine, remains the most effective treatment for restoring dopamine levels in the parkinsonian brain [4, 5]. However, due to extensive peripheral enzymatic metabolism by aromatic l-amino acid decarboxylase (AADC) and catechol-O-methyltransferase (COMT), only a fraction of the levodopa dose reaches the brain [6,7,8].
The COMT enzyme was first described in 1958 by Julius Axelrod and is responsible for the metabolism of compounds that contain a catechol structure, such as levodopa and dopamine [8, 9]. It also methylates other hydroxylated metabolites and catechol compounds in the diet [8]. COMT catalyses the transmission of a methyl group from S-adenosyl-l-methionine (SAMe) to a hydroxyl group of a catechol substrate in the presence of magnesium. The enzyme is widely distributed throughout the body with the liver containing the highest COMT activity [9]. Membrane-bound COMT (MB-COMT) and the soluble isoform of COMT, S-COMT, are the two major isoforms of the enzyme [10]. S-COMT is dominant in the peripheral tissue, and MB-COMT is mainly found in the brain.
COMT inhibitors are used in the symptomatic treatment of Parkinson’s disease where the peripheral inhibition of the O-methylation of levodopa increases the availability of levodopa for uptake into the brain [9, 11]. New-generation COMT inhibitors, such as the nitrocatechol COMT inhibitors (e.g. entacapone or tolcapone), are thus frequently used as adjunct to levodopa in Parkinson’s disease [12]. The clinical value of nitrocatechol COMT inhibitors is sometimes limited by adverse effects such as diarrhoea, hepatitis and neurological reactions, particularly for tolcapone [8].
Based on these observations, a series of 3-hydroxypyridin-4-ones were selected for investigation as potential COMT inhibitors. The 3-hydroxypyridin-4-one scaffold was chosen since the COMT inhibitory potential of several compounds from this class has been illustrated [13, 14]. 3-Hydroxypyridin-4-one is an isostere of the catechol ring, but is normally not O-methylated by the enzyme (Fig. 1). Further, it has been illustrated that non-nitrocatechol COMT inhibitors can be MB-COMT specific, which may be beneficial when considering that the peripheral side effects of COMT inhibitors are mostly related to their dopaminergic effects [13]. 3-Hydroxypyridin-4-ones may have three advantages over traditional nitrocatechol inhibitors: (1) Several derivatives have been shown to be orally active and are efficiently absorbed from the gastrointestinal tract [15]; (2) due to the presence of excessive iron deposits in the basal ganglia of the brain that have been associated with the aetiology of Parkinson’s disease [16], it may be of clinical benefit that 3-hydroxypyridin-4-ones display iron chelating activity [15, 17], which may reduce oxidative damage to susceptible neurons; and (3) analgesic effects of certain 3-hydroxypyridin-4-ones have further been illustrated in previous studies, indicating similar potency compared to aspirin and similar anti-inflammatory activity compared to indomethacin [18]. This may be beneficial for neuroinflammation, which has been implicated in neurodegeneration in Parkinson’s disease [19].
We thus report the synthesis of ten 3-hydroxypyridin-4-ones (1–10) and their evaluation as potential inhibitors of COMT (Table 1) [20]. Such compounds may represent useful agents for the treatment of Parkinson’s disease with improved safety profiles compared to nitrocatechol COMT inhibitors. This study explored different substituents on the pyridine nitrogen with simple aromatic and aliphatic substitution (1, 6 and 7), chain elongation and increasing flexibility (2, 3, 4 and 5), as well as halogen and methyl substitution on the side chain phenyl ring (8, 9 and 10).
Results and discussion
Chemistry
The 3-hydroxypyridin-4-ones were synthesised by a method described in the literature, whereby maltol was refluxed with a suitable primary amine in an acidic environment with ethanol as co-solvent (Fig. 2) [21]. After removal of an oily residue, the product precipitated from the reaction solution and was collected by filtration and purified by recrystallisation from hot methanol. Compounds 6 and 7 differed in work-up and were collected by extraction with ethyl acetate and purified by column chromatography. The synthesised compounds were obtained in yields of 4–12%. Characterisation of the compounds was carried out by NMR spectroscopy, IR spectroscopy and mass spectrometry. The purities of the compounds were estimated by HPLC analyses. It was confirmed that the purities (90–99%) of the 3-hydroxypyridin-4-one derivatives were acceptable for these compounds to be subjected to biological evaluation. Structures were confirmed using 1H NMR, 13C NMR, DEPT90 and DEPT135 spectra. Two-dimensional (2D) spectroscopic data were obtained by analysis of 1H–1H correlation spectroscopy (COSY), heteronuclear single-quantum correlation spectroscopy (HSQC) and heteronuclear multi-bond correlation spectroscopy (HMBC) data. Assignments were based on analysis of 1D spectra in conjunction with the 2D spectra (COSY, HSQC and HMBC) of these compounds (supplementary material).
General synthetic route for the synthesis of 3-hydroxypyridin-4-one derivatives 1–10 by a single-step synthetic pathway. Key: (a) HCl, H2O, ethanol, 72 h, 110 °C [21]
COMT inhibition
To determine whether the synthesised 3-hydroxypyridin-4-ones are in vitro inhibitors of COMT, commercially available COMT from porcine liver was used as enzyme source. A modified procedure reported in the literature was validated and used to measure COMT activity [22]. For this purpose, (−)-norepinephrine served as enzyme substrate, and the formation of the O-methylated product, normetanephrine, was quantified by reversed-phase high-performance liquid chromatography (HPLC) with fluorescence detection. A Km value of 379 µM was estimated for the methylation of (−)-norepinephrine by COMT, which is similar to that reported in the literature of 488 µM [22]. The determination of IC50 values should be carried out with the substrate concentration approximately equal to Km, and a typical enzyme reaction thus contained (−)-norepinephrine (250 µM), SAMe, MgCl2 and the test inhibitors. The reactions were initiated with the addition of the COMT enzyme, and after 60-min incubation, the reactions were terminated with perchloric acid. Following centrifugation, the supernatant fractions were analysed by HPLC for normetanephrine content. By measuring COMT activity in the presence of various concentrations of the test inhibitors, sigmoidal plots of enzyme catalytic rate versus the logarithm of inhibitor concentration were constructed from which the IC50 values were determined (Fig. 3).
The 3-hydroxypyridin-4-one derivatives exhibited COMT inhibitory activity with 2 and 10 as the most potent COMT inhibitors of the series with IC50 values of 4.55 μM and 5.76 μM, respectively (Table 1). Although active, these 3-hydroxypyridin-4-one derivatives are not as potent as the reference inhibitors, entacapone (IC50 = 0.00047 µM) and tolcapone (IC50 = 0.0068 µM). They may, however, serve as leads for the development of non-nitrocatechol COMT inhibitors with better side effect profiles [13]. Further investigation in this regard is required. Based on the IC50 values, preliminary structure–activity relationships (SARs) were derived for the inhibition of COMT by the 3-hydroxypyridin-4-ones: (1) Phenyl substitution of the 3-hydroxypyridin-4-one moiety (1) resulted in a higher IC50 value (and thus lower inhibition potency) compared to benzyl substitution (2). Benzyl substitution of the 3-hydroxypyridin-4-one moiety also resulted in improved COMT inhibition compared to phenylethyl (3), phenylpropyl (4) and phenylbutyl (5) substitution. This showed that chain elongation of the substituent reduces COMT inhibition potency and that the methylene (CH2) linker between the 3-hydroxypyridin-4-one moiety and phenyl ring was more optimal than the other linkers considered [(CH2)2, (CH2)3 and (CH2)4]. When comparing phenyl (1) substitution of the 3-hydroxypyridin-4-one moiety to cyclohexyl substitution (6), the aromatic substituent yielded slightly more favourable inhibition than the aliphatic substituent. Compared to cyclohexyl substitution (6), a reduction in COMT inhibition potency was observed for cyclopentyl substitution (7). In fact, 7 was the weakest COMT inhibitor of the series of 3-hydroxypyridin-4-one derivatives. When comparing the phenyl-substituted derivative (1) with the derivatives containing chlorine on the phenyl ring, compounds 8 and 9, it was apparent that chlorine substitution on the meta and para positions of the phenyl ring did not significantly affect COMT inhibition potency. Similarly, when the benzyl-substituted derivative 2 was substituted with a methyl substituent on the para position of the phenyl ring (to yield 10), COMT inhibitory activity remained relatively unchanged. Benzyl substitution of the 3-hydroxypyridin-4-one moiety (2 and 10) thus yielded the most potent COMT inhibitory activity among the compounds evaluated. The reference inhibitors, entacapone (IC50 = 0.00047 µM) and tolcapone (IC50 = 0.0068 µM), proved to be highly potent COMT inhibitors.
Molecular modelling
In an attempt to determine potential binding orientations and interactions of the 3-hydroxypyridin-4-ones with the COMT active site, molecular docking was carried out. The protein model of human COMT complexed with 3,5-dinitrocatechol was obtained from the Protein Data Bank (PDB code: 3BWM) [23]. The catechol binding site of COMT presents as a shallow cleft on the protein surface. It is defined by Met40, Leu198, Tyr200 as well as the “gatekeeper” residues Trp38 and Pro174, which ensures the correct orientation of the substrate for methylation [23]. Mg2+, which is a co-factor for the methylation reaction, is octahedrally coordinated to the side chains of Asp141 and Asp169, Asn170, the two hydroxy groups of the catechol substrate, and a water molecule [23]. Although not shown, the 1-hydroxy group of 3,5-dinitrocatechol is within hydrogen bonding distance to Glu199 and Asn170 in the shallow catechol binding site, while the 2-hydroxy and 3-nitro groups are within hydrogen bond distance to Lys144 (Fig. 4). The potential for hydrogen bonding and coordination with the Mg2+ ion underscores the importance of the catechol structure for binding to COMT.
Molecular docking was carried out according to the previously reported protocol with the CDOCKER application of the Discovery Studio [24]. The protein models were prepared by first calculating the pKa values and protonation states of the amino acid residues, which was followed by an energy minimisation with the protein backbone constrained. The structures of the ligands (3,5-dinitrocatechol and compound 2) were drawn in Discovery Studio, and after docking with CDOCKER, the docked orientations were refined using in situ ligand minimisation. Among the ten solutions generated, solutions existed for each ligand where the catechol hydroxyl groups of 3,5-dinitrocatechol or the 3-hydroxypyridin-4-one system of 2 are correctly placed for coordination with the Mg2+. As these are most probable, these solutions were selected and are presented here. The accuracy of the docking procedure was evaluated by redocking the co-crystallized ligand, 3,5-dinitrocatechol, into the active site of COMT. The root mean square deviation (RMSD) of the selected docked orientation from the position of the co-crystallised ligand was calculated to be 0.418 Å. RMSD values < 1.5 Å are considered to be successful, and this protocol was thus deemed appropriate for docking experiments with COMT [25].
The result of the docking study shows that the 3-hydroxypyridin-4-one system of 2 is correctly placed for coordination with the Mg2+ (Fig. 5). Although not shown, the 3-hydroxy group is within hydrogen bond distance from Asp141 and Lys144. The 2-methyl group overlaps with the 3-nitro group of 3,5-dinitrocatechol. The 1-benzyl side chain projects towards the exterior of the enzyme and mainly undergoes van der Waals interactions with Trp38 and Trp143. It may be concluded that compound 2 has the ability to adopt a similar orientation to 3,5-dinitrocatechol within the COMT active site and forms the appropriate polar interactions. The molecular reason for its lower potency compared to the nitrocatechol inhibitors, however, is not apparent. Based on the similarity of binding and interaction network between 3,5-dinitrocatechol and 2, it may be postulated that with appropriate structure modification, the COMT inhibition potencies of 3-hydroxypyridin-4-one may be significantly improved.
Conclusion
This study investigated a series of 3-hydroxypyridin-4-one derivatives as potential inhibitors of COMT. Benzyl substitution of the 3-hydroxypyridin-4-one moiety (2 and 10) yielded the most potent COMT inhibition of the series, with the most potent inhibitor exhibiting an IC50 value of 4.55 µM. Although the 3-hydroxypyridin-4-ones are relatively weak COMT inhibitors compared to the nitrocatechol compounds currently used, they may act as leads for the development of non-nitrocatechol COMT inhibitors for the treatment of Parkinson’s disease. In this respect, 3-hydroxypyridin-4-ones may exhibit other pharmacological actions that are relevant to neurodegenerative disorders (e.g. iron chelating, antioxidative and anti-inflammatory activities).
Experimental section
Chemicals and instrumentation
Reagents and solvents were obtained from Sigma-Aldrich and were used without any further purification. Deuterated dimethylsulfoxide (DMSO-d6) was used for nuclear magnetic resonance (NMR) spectroscopy and was purchased from Merck. A Bruker Avance III 600 spectrometer was used to record proton (1H) and carbon (13C) NMR spectra at 600 MHz and 150 MHz, respectively. To process and analyse the NMR data, MestReNova2 was used. Data reported included integration (e.g. 1H), multiplicity and coupling constants (J) given in hertz (Hz). Chemical shifts (δ), in parts per million (ppm), were referenced to the residual solvent (DMSO-d6) signal at 2.5 ppm for 1H NMR and 39.5 ppm for 13C NMR. Spin multiplicities were presented as s (singlet), br s (broad singlet), d (doublet), br d (broad doublet), ddd (doublet of doublet of doublets), t (triplet), br t (broad triplet), p (pentet) or m (multiplet). Note that the OH proton of the 3-hydroxy group was not observed in the 1H NMR spectra. A Bruker micrOTOF-Q II mass spectrometer functioning in atmospheric-pressure chemical ionisation (APCI) mode was used to record high-resolution mass spectra (HRMS). Infrared spectra were obtained using an Alpha Bruker Fourier Transform Infrared (FTIR) spectrometer. Opus 7.0.129 software was used to process and record IR data, reporting absorption bands in cm−1. TLC (thin-layer chromatography) was used to monitor the progress of the reactions and to determine whether the reactions were complete. The reagents and products were dissolved in methanol, applied to silica gel 60 aluminium-coated TLC sheets (Merck) and developed in an appropriate mobile phase. Ethyl acetate was used as eluent during TLC of compounds 1, 2, 8 and 9, while a mixture of methanol and ethyl acetate (9:1) was used as mobile phase for compounds 3–7 and 10. The developed TLC sheets were visualised under an UV lamp at a wavelength of 254 nm. HPLC (high-performance liquid chromatography) analyses were performed to estimate the purities of the synthesised compounds. These were conducted with an Agilent 1100 HPLC system equipped with a quaternary pump and an Agilent 1100 series diode array detector. HPLC-grade acetonitrile (Merck) and Milli-Q water (Millipore) were used for the preparation of the mobile phase. A Venusil XBP C18 column (4.60 × 150 mm, 5 µm) was used as analytical column. Initially, the mobile phase consisted of 30% acetonitrile and 70% Milli-Q water and the flow rate was set to 1 mL/min. A solvent gradient programme was initiated at the beginning of each HPLC run. This was done by linearly increasing the composition of the acetonitrile in the mobile phase to 85% acetonitrile over a 5-min period. HPLC runs lasted 15 min, and a 5-min period was allowed for equilibration of the system. The test compounds were dissolved in acetonitrile to a concentration of 1 mM, and 20 µL of these solutions was injected into the HPLC system. The eluent was monitored at a wavelength of 254 nm.
Synthesis of 3-hydroxypyridin-4-one derivatives 1–5, 8–10
The synthesis of the 3-hydroxypyridin-4-one derivatives was achieved in a single-step synthesis and is based on a modification of the procedure reported in the literature [21]. Commercially available maltol (20 mmol, 1 equiv.) was heated under reflux at 110 °C with a suitable primary amine (30 mmol, 1.5 equiv.) in the presence of HCl (32%, 0.8 mL), water (36 mL) and ethanol (4 mL) for 72 h. TLC analysis of the reaction progress indicated that it was necessary to add an additional 10 mmol (0.5 equiv.) of the primary amine to complete the reaction. After reflux was completed, a dark oily residue was allowed to separate to the bottom of the round-bottom flask. While still hot, the light yellow clear top layer was decanted from the oily residue. The clear solution was covered and incubated overnight at room temperature during which a white solid precipitated from the solution. The precipitate was collected by filtration the following morning and allowed to air-dry overnight. Recrystallisation from hot methanol gave a white crystalline solid.
Synthesis of 3-hydroxypyridin-4-one derivatives 6 and 7
The synthesis of 6 and 7 follows the same general procedure as described for the 3-hydroxypyridin-4-one derivatives above, but differs in the work-up. After reflux was completed, the reactions did not yield dark oily residues, and no precipitate was obtained after cooling of the reactions. Instead, the resulting reaction mixture remained a clear red orange solution. After cold distilled water (20 mL) was added to the reaction, it was extracted to ethyl acetate (1 × 20 mL for 6; 2 × 30 mL for 7). The combined organic layers were dried over anhydrous magnesium sulphate and filtered, and the solvent was removed by rotary evaporation. The crude product was then allowed to air-dry to yield a red powder that was purified with silica gel column chromatography (methanol/ethyl acetate, 9:1).
3-Hydroxy-2-methyl-1-phenylpyridin-4-one ( 1 )
The title compound was prepared from maltol and aniline in a yield of 6.56% (132 mg): mp 223.3–223.9 °C (methanol) (lit. 221–222 °C [21]), white crystals. 1H NMR (600 MHz, DMSO-d6) δ 7.60–7.50 (m, 4H, H-6, H-3′/5′, H-4′), 7.48–7.43 (m, 2H, H-2′/6′), 6.22 (d, J = 7.3 Hz, 1H, H-5), 1.96 (s, 3H, CH3). 13C NMR (151 MHz, DMSO-d6) δ 169.7 (C-4), 145.1 (C-3), 141.6 (C-1′), 137.9 (C-6), 129.7 (C-3′/5′), 129.1 (C-4′), 128.8 (C-2), 127.0 (C-2′/6′), 111.0 (C-5), 13.4 (CH3). APCI-HRMS m/z: calcd for C12H12NO2 (MH+), 202.0863, found 202.0854; purity (HPLC): 99% (254 nm). FTIR v/cm−1: 3197 (broad OH), 1626 (C=O), 1578 (C=C), 1235 (C–N).
1-Benzyl-3-hydroxy-2-methylpyridin-4-one ( 2 )
The title compound was prepared from maltol and benzylamine in a yield of 4.88% (105 mg): mp 205.6–207.1 °C (methanol), white crystals. 1H NMR (600 MHz, DMSO-d6) δ 7.75 (d, J = 7.3 Hz, 1H, H-6), 7.37 (br t, J = 7.6 Hz, 2H, H-3′/5′), 7.33–7.27 (m, 1H, H-4′), 7.06 (br d, J = 7.0 Hz, 2H, H-2′/6′), 6.21 (d, J = 7.2 Hz, 1H, H-5), 5.25 (s, 2H, H-1″), 2.12 (s, 3H, CH3). 13C NMR (151 MHz, DMSO-d6) δ 169.3 (C-4), 145.8 (C-3), 138.6 (C-6), 137.1 (C-1′), 129.1 (C-2), 129.0 (C-3′/5′), 127.7 (C-4′), 126.1 (C-2′/6′), 110.9 (C-5), 56.0 (C-1″), 11.6 (CH3). APCI-HRMS m/z: calcd for C13H14NO2 (MH+), 216.1019, found 216.1003; purity (HPLC): 99% (254 nm). FTIR v/cm−1: 3175 (broad OH), 1626 (C=O), 1574 (C=C), 1234 (C–N).
3-Hydroxy-2-methyl-1-(2-phenylethyl)pyridin-4-one ( 3 )
The title compound was prepared from maltol and 2-phenyl-1-ethylamine in a yield of 6.42% (147 mg): mp 159.4–160.8 °C (methanol), white crystals. 1H NMR (600 MHz, DMSO-d6) δ 7.45 (d, J = 7.3 Hz, 1H, H-6), 7.29 (br t, J = 7.3 Hz, 2H, H3′/5′), 7.26–7.17 (m, 3H, H-2′/6′, H-4′), 6.06 (d, J = 7.2 Hz, 1H, H-5), 4.14 (t, J = 7.5 Hz, 2H, H-1″), 2.95 (t, J = 7.5 Hz, 2H, H-2″), 2.25 (s, 3H, CH3). 13C NMR (151 MHz, DMSO-d6) δ 168.9 (C-4), 145.4 (C-3), 137.52 (C-6), 137.46 (C-1′), 129.0 (C-2′/6′ or C-3′/5′), 128.7 (C-2), 128.5 (C-2′/6′ or C-3′/5′), 126.7 (C-4′), 110.6 (C-5), 53.9 (C-1″), 36.3 (C-2″), 11.3 (CH3). APCI-HRMS m/z: calcd for C14H16NO2 (MH+), 230.1176, found 230.1186; purity (HPLC): 99% (254 nm). FTIR v/cm−1: 3188 (broad OH), 1625 (C=O), 1558 (C=C), 1231 (C–N).
3-Hydroxy-2-methyl-1-(3-phenylpropyl)pyridin-4-one ( 4 )
The title compound was prepared from maltol and 3-phenyl-1-propylamine in a yield of 12.92% (294 mg): mp 157.2–159.3 °C (methanol), white crystals. 1H NMR (600 MHz, DMSO-d6) δ 7.57 (d, J = 7.3 Hz, 1H, H-6), 7.31–7.25 (m, 2H, H-3′/5′), 7.25–7.16 (m, 3H, H-2′/6′, H-4′), 6.13 (d, J = 7.2 Hz, 1H, H-5), 3.94 (t, J = 7.4 Hz, 2H, H-1″), 2.60 (t, J = 7.8 Hz, 2H, H-3″), 2.23 (s, 3H, CH3), 1.94 (p, J = 7.8 Hz, 2H, H-2″). 13C NMR (151 MHz, DMSO-d6) δ 168.9 (C-4), 145.5 (C-3), 140.8 (C-1′), 137.5 (C-6), 128.6 (C-2), 128.4 (C-2′/6′ or C-3′/5′), 128.2 (C-2′/6′ or C-3′/5′), 126.0 (C-4′), 110.7 (C-5), 52.4 (C-1″), 31.8 (C-2″, C-3″), 11.3 (CH3). APCI-HRMS m/z: calcd for C15H18NO2 (MH+), 244.1332, found 244.1331; purity (HPLC): 99% (254 nm). FTIR v/cm−1: 3118 (broad OH), 1624 (C=O), 1572 (C=C), 1219 (C–N).
3-Hydroxy-2-methyl-1-(4-phenylbutyl)pyridin-4-one ( 5 )
The title compound was prepared from maltol and 4-phenyl-1-butylamine in a yield of 4.90% (126 mg): mp 171.6–173.7 °C (methanol), white crystals. 1H NMR (600 MHz, DMSO-d6) δ 7.55 (d, J = 7.3 Hz, 1H, H-6), 7.30–7.24 (m, 2H, H-3′/5′), 7.21–7.14 (m, 3H, H-2′/H-6′, H-4′), 6.09 (d, J = 7.2 Hz, 1H, H-5), 3.94 (t, J = 7.3 Hz, 2H, H-1″), 2.59 (t, J = 7.6 Hz, 2H, H-4″), 2.25 (s, 3H, CH3), 1.68–1.50 (m, 4H, H-2″, H-3″). 13C NMR (151 MHz, DMSO-d6) δ 168.8 (C-4), 145.5 (C-3), 141.8 (C-1′), 137.6 (C-6), 128.5 (C-2), 128.32 (C-2′/6′ or C-3′/5′), 128.28 (C-2′/6′ or C-3′/5′), 125.8 (C-4′), 110.5 (C-5), 52.7 (C-1″), 34.6 (C-4″), 29.8 (C-2″ or C-3″), 27.7 (C-2″ or C-3″), 11.3 (CH3). APCI-HRMS m/z: calcd for C16H20NO2 (MH+), 258.1489, found 258.1481; purity (HPLC): 98% (254 nm). FTIR v/cm−1: 3079 (broad OH), 1626 (C=O), 1567 (C=C), 1223 (C–N).
1-Cyclohexyl-3-hydroxy-2-methylpyridin-4-one ( 6 )
The title compound was prepared from maltol and cyclohexylamine in a yield of 6.86% (142 mg): mp 191.6–202.8 °C (methanol), red solid. 1H NMR (600 MHz, DMSO-d6) δ 7.67 (br s, 1H, H-6), 6.15 (br s, 1H, H-5), 4.02 (br s, 1H, H-1′), 2.32 (s, 3H, CH3), 2.09–0.74 (m, 10H, H-2′/6′, H-3′/5′, H-4′). 13C NMR (151 MHz, DMSO-d6) δ 168.6 (C-4), 145.3 (C-3), 133.3 (C-6), 128.8 (C-2), 110.8 (C-5), 59.0 (C-1′), 32.5 (C-2′/6′), 25.3 (C-3′/5′), 24.6 (C-4′), 11.3 (CH3). APCI-HRMS m/z: calcd for C12H18NO2 (MH+), 208.1332, found 208.1344; purity (HPLC): 92% (254 nm). FTIR v/cm−1: 3081 (broad OH), 1619 (C=O), 1566 (C=C), 1216 (C–N).
1-Cyclopentyl-3-hydroxy-2-methylpyridin-4-one ( 7 )
The title compound was prepared from maltol and cyclopentylamine in a yield of 8.39% (162 mg): mp 51.4–51.5 °C (methanol), red solid. 1H NMR (600 MHz, DMSO-d6) δ 7.62 (br s, 1H, H-6), 6.17 (br s, 1H, H-5), 4.61 (br s, 1H, H-1′), 2.46–1.28 (m, 11H, H-2′/5′, H-3′/4′, CH3). 13C NMR (151 MHz, DMSO-d6) δ 168.7 (C-4), 145.4 (C-3), 133.1 (C-6), 129.3 (C-2), 111.1 (C-5), 60.9 (C-1′), 32.5 (C-2′/5′), 23.7 (C-3′/4′), 11.7 (CH3). APCI-HRMS m/z: calcd for C11H16NO2 (MH+), 194.1176, found 194.1174; purity (HPLC): 91% (254 nm). FTIR v/cm−1: 3078 (broad OH), 1621 (C=O), 1562 (C=C), 1212 (C–N).
1-(3-Chlorophenyl)-3-hydroxy-2-methylpyridin-4-one ( 8 )
The title compound was prepared from maltol and 3-chloroaniline in a yield of 5.06% (119 mg): mp 185.6–186.2 °C (methanol), white solid. 1H NMR (600 MHz, DMSO-d6) δ 7.68 (br t, J = 2.0 Hz, 1H, H-2′), 7.64–7.55 (m, 3H, H-6′, H-5′, H-6), 7.46 (ddd, J = 7.7, 2.1, 1.2 Hz, 1H, H-4′), 6.23 (d, J = 7.3 Hz, 1H, H-5), 1.97 (s, 3H, CH3). 13C NMR (151 MHz, DMSO-d6) δ 169.8 (C-4), 145.1 (C-3), 142.7 (C-1′), 137.9 (C-6), 133.8 (C-3′), 131.2 (C-6′), 129.4 (C-5′), 128.9 (C-2), 127.3 (C-2′), 126.2 (C-4′), 111.2 (C-5), 13.4 (CH3). APCI-HRMS m/z: calcd for C12H11ClNO2 (MH+), 236.0473, found 236.0446; purity (HPLC): 94% (254 nm). FTIR v/cm−1: 3057 (broad OH), 1627 (C=O), 1561 (C=C), 1245 (C–N).
1-(4-Chlorophenyl)-3-hydroxy-2-methylpyridin-4-one ( 9 )
The title compound was prepared from maltol and 4-chloroaniline in a yield of 5.09% (120 mg): mp 298.1–298.4 °C (methanol), white solid. 1H NMR (600 MHz, DMSO-d6) δ 7.63 (d, J = 8.7 Hz, 2H, H-2′/6′ or H-3′/5′), 7.55 (d, J = 7.3 Hz, 1H, H-6), 7.51 (d, J = 8.7 Hz, 2H, H-2′/6′ or H-3′/5′), 6.22 (d, J = 7.3 Hz, 1H, H-5), 1.96 (s, 3H, CH3). 13C NMR (151 MHz, DMSO-d6) δ 169.8 (C-4), 145.1 (C-3), 140.4 (C-1′), 137.9 (C-6), 133.7 (C-4′), 129.7 (C-2′/6′ or C-3′/5′), 129.1 (C-2′/6′ or C-3′/5′), 128.7 (C-2), 111.1 (C-5), 13.4 (CH3). APCI-HRMS m/z: calcd for C12H11ClNO2 (MH+), 236.0473, found 236.0465; purity (HPLC): 90% (254 nm). FTIR v/cm−1: 3047 (broad OH), 1622 (C=O), 1582 (C=C), 1233 (C–N).
3-Hydroxy-2-methyl-1-(4-methylbenzyl)pyridin-4-one ( 10 )
The title compound was prepared from maltol and 4-methylbenzylamine in a yield of 7.68% (176 mg): mp 213.7–226.0 °C (methanol), white solid. 1H NMR (600 MHz, DMSO-d6) δ 7.73 (d, J = 7.3 Hz, 1H, H-6), 7.18 (d, J = 7.8 Hz, 2H, H-3′/5′), 6.96 (d, J = 7.9 Hz, 2H, H-2′/6′), 6.19 (d, J = 7.2 Hz, 1H, H-5), 5.19 (s, 2H, H-1″), 2.27 (s, 3H, CH3 benzyl), 2.12 (s, 3H, CH3 pyridone). 13C NMR (151 MHz, DMSO-d6) δ 169.2 (C-4), 145.8 (C-3), 138.5 (C-6), 137.0 (C-1′), 134.0 (C-4′), 129.5 (C-3′/5′), 129.0 (C-2), 126.1 (C-2′/6′), 110.8 (C-5), 55.8 (C-1″), 20.7 (CH3 benzyl), 11.6 (CH3 pyridone). APCI-HRMS m/z: calcd for C14H16NO2 (MH+), 230.1176, found 230.1166; purity (HPLC): 93% (254 nm). FTIR v/cm−1: 3203 (broad OH), 1627 (C=O), 1576 (C=C), 1239 (C–N).
COMT inhibition studies
Purified COMT from porcine liver (≥ 150 units/mg), normetanephrine hydrochloride and SAMe were obtained from Sigma-Aldrich. The enzyme stock solution was prepared by dissolving the purified COMT (750 units/5 mL) in sodium phosphate buffer (25 mM, pH 7.8) containing dithiothreitol (0.5 mM). The test inhibitors were prepared in DMSO and added to the reactions at concentrations of 0.1–100 µM, while the reference inhibitors, tolcapone and entacapone, were evaluated at 0.0003–1 µM. The final DMSO concentration in the reactions was 4%. The enzyme reactions (125 µL) contained MgCl2 (2 mM; 10 µL), (−)-norepinephrine (250 µM; 35 µL), SAMe (200 µM, 25 µL) and the test inhibitor (5 µL). After the reactions were pre-incubated at 37 °C in a water bath for at least 10 min, a 50-µL aliquot of the enzyme stock solution was added. The reactions were incubated for 60 min and subsequently terminated by adding 12.5 µL perchloric acid (1 M). The samples were centrifuged at 16,000×g for 10 min, and the supernatants were analysed by HPLC. The peak areas of normetanephrine were recorded, and the corresponding concentrations of normetanephrine formed were calculated using a calibration curve (1–30 µM). Using the Prism 5 (GraphPad) software package, the data were fitted to the equation for one-site competition to yield a sigmoidal curve of concentration of normetanephrine formed versus the logarithm of inhibitor concentration. IC50 values were estimated from these plots. For each test inhibitor, the IC50 values were determined in triplicate and expressed as mean ± SD.
For the HPLC analysis of normetanephrine, an Agilent 1100 HPLC system equipped with a Shimadzu RF-551 fluorescence detector was used. A Phenomenex USP L1 Luna C18 column (250 × 4.6 mm, 5 μm) was used for chromatographic separation. A mixture of an aqueous phase and an organic phase was used to prepare the mobile phase. The aqueous phase consisted of sodium phosphate buffer (10 mM, pH 7.0), boric acid (80 mM) and sodium 1-hexanesulfonate (4 mM). The organic phase consisted of acetonitrile, and the aqueous and organic phases were mixed in a ratio of 85:15. The HPLC system was set to a flow rate of 1 mL/min with a sample injection volume of 20 μL, and fluorescence detection was carried out at an excitation wavelength of 283 nm and an emission wavelength of 315 nm. A retention time of ± 4.7 min was recorded for normetanephrine [22].
Molecular docking studies
Discovery Studio 3.1 (Accelrys) was used to carry out molecular docking. The protein model of human COMT was obtained from the Protein Data Bank (PDB code: 3BWM) [23]. The default ‘prepare protein’ option was used to protonate the model, and the pKa values and protonation states of the ionisable amino acids were calculated at pH 7.4. The valences of the cofactors and co-crystallised ligand (3,5-dinitrocatechol) were corrected, and the protein models were automatically typed with the Momany and Rone CHARMm forcefield. The Smart Minimizer algorithm was used to minimise the protein structure using a fixed backbone constraint and the generalised born approximation with molecular volume (GBMV) as solvent model (50,000 steps). The crystal water molecules were deleted, and the binding site was defined from the position of the co-crystallised ligand. The structures of 2 and 3,5-dinitrocatechol were constructed in Discovery Studio and prepared using the ‘prepare ligands’ function. The Momany and Rone CHARMm forcefield was used to assigned atom potential types and partial charges to the ligands. The prepared ligands were then docked into the active site of the COMT model using the CDOCKER algorithm, allowing for ten random ligand conformations and the heating target temperature set to 700 K in full potential mode. The docking solutions were finally refined using in situ ligand minimisation with the Smart Minimizer algorithm. The illustrations were prepared with the PyMOL molecular graphics system [26].
References
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909. https://doi.org/10.1016/S0896-6273(03)00568-3
Lees AJ, Hardy J, Revesz T (2009) Parkinson’s disease. Lancet 373:2055–2066. https://doi.org/10.1016/S0140-6736(09)60492-X
LeWitt PA, Taylor DC (2008) Protection against Parkinson’s disease progression: clinical experience. Neurotherapeutics 5:210–225. https://doi.org/10.1016/j.nurt.2008.01.007
Brichta L, Greengard P, Flajolet M (2013) Advances in the pharmacological treatment of Parkinson’s disease: targeting neurotransmitter systems. Trends Neurosci 36:543–554. https://doi.org/10.1016/j.tins.2013.06.003
Freitas ME, Ruiz-Lopez M, Fox SH (2016) Novel levodopa formulations for Parkinson’s disease. CNS Drugs 30:1079–1095. https://doi.org/10.1007/s40263-016-0386-8
Contin M, Martinelli P (2010) Pharmacokinetics of levodopa. J Neurol 257:S253–S261. https://doi.org/10.1007/s00415-010-5728-8
Nutt JG, Fellman JH (1984) Pharmacokinetics of levodopa. Clin Neuropharmacol 7:35–49. https://doi.org/10.1097/00002826-198403000-00002
Kaakkola S (2000) Clinical pharmacology, therapeutic use and potential of COMT inhibitors in Parkinson’s disease. Drugs 59:1233–1250. https://doi.org/10.2165/00003495-200059060-00004
Mannisto PT, Kaakkola S (1999) Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev 51:593–628
Kiss LE, Soares-da-Silva P (2014) Medicinal chemistry of catechol O-methyltransferase (COMT) inhibitors and their therapeutic utility. J Med Chem 57:8692–8717. https://doi.org/10.1021/jm500572b
Silva T, Mohamed T, Shakeri A, Rao PPN, Martinez-Gonzalez L, Perez DI, Martinez A, Valente MJ, Garrido J, Uriarte E, Serrao P, Soares-da-Silva P, Remiao F, Borges F (2016) Development of blood–brain barrier permeable nitrocatechol-based catechol O-methyltransferase inhibitors with reduced potential for hepatotoxicity. J Med Chem 59:7584–7597. https://doi.org/10.1021/acs.jmedchem.6b00666
Lees AJ (2008) Evidence-based efficacy comparison of tolcapone and entacapone as adjunctive therapy in Parkinson’s disease. CNS Neurosci Ther 14:83–93. https://doi.org/10.1111/j.1527-3458.2007.00035.x
Robinson RG, Smith SM, Wolkenberg SE, Kandebo M, Yao LH, Gibson CR, Harrison ST, Polsky-Fisher S, Barrow JC, Manley PJ, Mulhearn JJ, Nanda KK, Schubert JW, Trotter BW, Zhao ZJ, Sanders JM, Smith RF, McLoughlin D, Sharma S, Hall DL, Walker TL, Kershner JL, Bhandari N, Hutson PH, Sachs NA (2012) Characterization of non-nitrocatechol pan and isoform specific catechol-O-methyltransferase inhibitors and substrates. ACS Chem Neurosci 3:129–140. https://doi.org/10.1021/cn200109w
Borchardt RT (1973) Catechol O-methyltransferase. 4. In vitro inhibition by 3-hydroxy-4-pyrones, 3-hydroxy-2-pyridones, and 3-hydroxy-4-pyridones. J Med Chem 16:581–583. https://doi.org/10.1021/jm00263a047
Rai BL, Liu ZD, Liu DY, Lu SL, Hider RC (1999) Synthesis, physicochemical properties and biological evaluation of ester prodrugs of 3-hydroxypyridin-4-ones: design of orally active chelators with clinical potential. Eur J Med Chem 34:475–485. https://doi.org/10.1016/S0223-5234(99)80097-X
Zucca FA, Segura-Aguilar J, Ferrari E, Munoz P, Paris I, Sulzer D, Sarna T, Casella L, Zecca L (2017) Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog Neurobiol 155:96–119. https://doi.org/10.1016/j.pneurobio.2015.09.012
Liu ZD, Liu DY, Lu SL, Hider RC (1999) Synthesis, physicochemical properties and biological evaluation of aromatic ester prodrugs of 1-(2′-hydroxyethyl)-2-ethyl-3-hydroxypyridin-4-one (CP102): orally active iron chelators with clinical potential. J Pharm Pharmacol 51:555–564. https://doi.org/10.1211/0022357991772655
Ozturk G, Erol DD, Uzbay T, Aytemir MD (2001) Synthesis of 4(1H)-pyridinone derivatives and investigation of analgesic and antiinflammatory activities. Farmaco 56:251–256. https://doi.org/10.1016/S0014-827x(01)01083-7
Guzman-Martinez L, Maccioni RB, Andrade V, Navarrete LP, Pastor MG, Ramos-Escobar N (2019) Neuroinflammation as a common feature of neurodegenerative disorders. Front Pharmacol. https://doi.org/10.3389/fphar.2019.01008
De Beer J (2015) Design, synthesis and evaluation of 3-hydroxypyridin-4-ones as inhibitors of catechol-O-methyltransferase. Dissertation, North-West University, Potchefstroom, South Africa
Fassihi A, Abedi D, Saghaie L, Sabet R, Fazeli H, Bostaki G, Deilami O, Sadinpour H (2009) Synthesis, antimicrobial evaluation and QSAR study of some 3-hydroxypyridine-4-one and 3-hydroxypyran-4-one derivatives. Eur J Med Chem 44:2145–2157. https://doi.org/10.1016/j.ejmech.2008.10.022
Aoyama N, Tsunoda M, Imai K (2005) Improved assay for catechol-O-methyltransferase activity utilizing norepinephrine as an enzymatic substrate and reversed-phase high-performance liquid chromatography with fluorescence detection. J Chromatogr A 1074:47–51. https://doi.org/10.1016/j.chroma.2005.03.037
Rutherford K, Le Trong I, Stenkamp RE, Person VW (2008) Crystal structures of human 108V and 108M catechol O-methyltransferase. J Mol Biol 380:120–130. https://doi.org/10.1016/j.jmb.2008.04.040
Mostert S, Petzer A, Petzer JP (2015) Indanones as high-potency reversible inhibitors of monoamine oxidase. ChemMedChem 10:862–873. https://doi.org/10.1002/cmdc.201500059
Hevener KE, Zhao W, Ball DM, Babaoglu K, Qi JJ, White SW, Lee RE (2009) Validation of molecular docking programs for virtual screening against dihydropteroate synthase. J Chem Inf Model 49:444–460. https://doi.org/10.1021/ci800293n
DeLano WL (2002) The PyMOL molecular graphics system. DeLano Scientific, San Carlos
Acknowledgements
The NMR and MS spectra were recorded by André Joubert and Johan Jordaan of the Laboratory for Analytical Services (LAS) in the focus area Chemical Resource Beneficiation, respectively, while HPLC analyses were carried out by Jan du Preez of the Analytical Technology Laboratory (ATL) at the North-West University (NWU).
Funding
The financial assistance of the National Research Foundation (NRF) of South Africa [Grant specific unique Reference Numbers (UID) 85642, 96180] towards this research is hereby acknowledged. The Grantholders acknowledge that opinions, findings and conclusions or recommendations expressed in any publication generated by the NRF supported research are that of the authors and that the NRF accepts no liability whatsoever in this regard.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Beer, J., Petzer, J.P., Lourens, A.C.U. et al. Design, synthesis and evaluation of 3-hydroxypyridin-4-ones as inhibitors of catechol-O-methyltransferase. Mol Divers 25, 753–762 (2021). https://doi.org/10.1007/s11030-020-10053-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-020-10053-x