Abstract
Background
The accidental ingestion of the third larval stage of Anisakis can cause acute clinical symptoms, which are relieved via extraction of the larvae. Although this is a highly effective technique, it can only be practiced when the larvae are found in accessible areas of the gastrointestinal tract, and therefore instead the condition has often been treated using various different drugs.
Aims
This study evaluates the effectiveness of gastric acid secretion inhibitors (omeprazole and ranitidine), gastric mucosal protectants (sucralfate) and anthelmintics (mebendazole and flubendazole) in treating anisakiasis in Wistar rats.
Methods
Rats were infected with Anisakis-type I larvae and administered the drugs via a gastric probe. Data were recorded regarding the number of live and dead larvae, their location both within the animal and in its feces, and the presence of gastrointestinal lesions. Additionally, gastric pH was measured and histology performed.
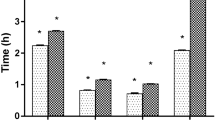
Results
While rats in all experimental groups exhibited lesions; those treated with ranitidine and mebendazole showed significantly fewer lesions (50% and 35% of rats exhibited lesions, respectively). Histological examination of the gastric lesions revealed infection-induced changes, but no significant differences were observed between the treated and untreated rats.
Conclusions
Mebendazole was found to be most efficacious in preventing gastrointestinal lesions, followed by ranitidine, which was the most effective antacid of those studied. Both these drugs could thus be considered as part of the conservative management of anisakiasis.


Similar content being viewed by others
References
Smith JW, Wootten R. Anisakis and anisakiasis. Adv Parasitol. 1978;16:93–163.
Chía N, Romero MC, Polo-Vico R, Gómez-Mateos M, Abattouy N, Valero A. Epidemiological study of Anisakis type I in blue whiting (Micromesistius poutassou) captured in northwestern Spain. Ars Pharm. 2010;51:829–834.
Molina-Fernández D, Malagón D, Gómez-Mateos M, Benítez R, Martín-Sánchez J, Adroher FJ. Fishing area and fish size as risk factors of Anisakis infection in sardines (Sardina pilchardus) from Iberian waters, southwestern Europe. Int J Food Microbiol. 2015;203:27–34.
Levsen A, Cipriani P, Mattiucci S, et al. Anisakis species composition and infection characteristics in Atlantic mackerel, Scomber scombrus, from major European fishing grounds—reflecting changing fish host distribution and migration pattern. Fish Res. 2017;202:112–121.
Van Thiel P. Larva migrans visceralis. Ned Tijdschr Geneeskd. 1960;104:1104–1107.
Chai JY, Darwin Murrell K, Lymbery AJ. Fish-borne parasitic zoonoses: status and issues. Int J Parasitol. 2005;35:1233–5124.
Mattiucci S, Paoletti M, Borrini F, et al. First molecular identification of the zoonotic parasite Anisakis pegreffii (Nematoda: Anisakidae) in a paraffin-embedded granuloma taken from a case of human intestinal anisakiasis in Italy. BMC Infect Dis. 2011;11:82.
Li SW, Shiao SH, Weng SC, Liu TH, Su KE, Chen CC. A case of human infection with Anisakis simplex in Taiwan. Gastrointest Endosc. 2015;82:757–758.
Lim H, Jung B, Cho J, Yooyen T, Shin E, Chai J. Molecular diagnosis of cause of anisakiasis, South Korea. Emerg Infect Dis. 2015;21:342–344.
Amir A, Ngui R, Lau YL, et al. Anisakiasis causing acute dysentery in Malaysia. Am J Trop Med Hyg. 2016;95:410–412.
Moneo I, Carballeda-Sangiao N, González-Muñoz M. New perspectives on the diagnosis of allergy to Anisakis spp. Curr Allergy Asthma Rep. 2017;17:27.
Bouwknegt M, Devleesschauwer B, Graham H, Robertson LJ, Van der Giessen JW. The Euro-Fbp workshop participants. Prioritisation of food-borne parasites in Europe, 2016. Euro Surveill. 2018. https://doi.org/10.2807/1560-7917.ES.2018.23.9.17-00161.
Bao M, Pierce GJ, Pascual S, et al. Assessing the risk of an emerging zoonosis of worldwide concern: anisakiasis. Sci Rep. 2017;7:43699.
Herrador Z, Daschner Á, Perteguer MJ, Benito A. Epidemiological scenario of anisakidosis in Spain based on associated hospitalizations: the tipping point of the iceberg. Clin Infect Dis. 2019;69:69–76.
Audicana MT, Kennedy MW. Anisakis simplex: from obscure infectious worm to inducer of immune hypersensitivity. Clin Microbiol Rev. 2008;21:360–379.
Morsy K, Badr AM, Abdel-Ghaffar F, El Deeb S, Ebead S. Pathogenic potential of fresh, frozen, and thermally treated Anisakis spp. type II (L3) (Nematoda: Anisakidae) after oral inoculation into wistar rats: a histopathological study. J Nematol. 2017;49:427–436.
Shimamura Y, Muwanwella N, Chandran S, Kandel G, Marcon N. Common symptoms from an uncommon infection: gastrointestinal anisakiasis. Can J Gastroenterol Hepatol. 2016;2016:5176502.
Shibata E, Ueda T, Akaike G, Saida Y. CT findings of gastric and intestinal anisakiasis. Abdom Imaging. 2014;39:257–261.
Takabayashi T, Mochizuki T, Otani N, Nishiyama K, Ishimatsu S. Anisakiasis presenting to the ED: clinical manifestations, time course, hematologic tests, computed tomographic findings, and treatment. Am J Emerg Med. 2014;32:1485–1489.
Fuchizaki U, Nishikawa M. Images in clinical medicine. Gastric Anisakiasis. N Engl J Med. 2016;375:e11.
Baron L, Branca G, Trombetta C, et al. Intestinal anisakidosis: histopathological findings and differential diagnosis. Pathol Res Pract. 2014;210:746–750.
Theodore E. Anisakiasis. In: Gerald L, John E, Raphael D, eds. Principles and Practice of Infectious Diseases. Philadelphia: Elsevier Churchill Livingstone; 2005:3295.
De la Fuente E, Agudo S, Blasco S, Cacho G, Fernández C. Casos clínicos: caso de anisakiasis gastroalérgica documentado endoscópicamente. Rev Esp Enferm Dig. 2013;105:245–380.
Nakata H, Takeda K, Nakayama T. Radiological diagnosis of acute gastric anisakiasis. Radiology. 1980;135:49–53.
Cocheton JJ, Cabou I, Lecomte I. Anisakiasis and Anisakis infections. Ann Med Interne. 1991;142:121–130.
Iglesias L, Valero A, Benítez R, Adroher FJ. In vitro cultivation of Anisakis simplex: pepsin increases survival and moulting from fourth larval to adult stage. Parasitology. 2001;123:285–291.
Henríquez-Santana A, Villafruela-Cives M. Anisakis: past, present and future. Med Clin. 2009;132:400–403.
Moore D, Girdwood R, Chiodini P. Treatment of anisakiasis with albendazole. Lancet. 2002;360:54.
Kim T, Lee B, Sohn W. Three clinical cases of cutaneous larva migrans. Korean J Parasitol. 2006;44:145–149.
Kim SH, Park CW, Kim SK, et al. A case of anisakiasis invading the stomach and the colon at the same time after eating anchovies. Clin Endosc. 2013;46:293–296.
Dziekonska-Rynko J, Rokicki J, Jablonowski Z. Effects of ivermectin and albendazole against Anisakis simplex in vitro and in guinea pigs. J Parasitol. 2002;88:395–398.
Arias-Diaz J, Zuloaga J, Vara E, Balibrea J, Balibrea JL. Efficacy of albendazole against Anisakis simplex larvae in vitro. Dig Liver Dis. 2006;38:24–26.
Lin RJ, Chen CY, Lee JD, Lu CM, Chung LY, Yen CM. Larvicidal constituents of Zingiber officinale (Ginger) against Anisakis simplex. Planta Med. 2010;76:1852–1858.
Lopieńska-Biernat E, Molcan T, Paukszto L, Jastrzębski JP, Myszczyński K. Modelling studies determing the mode of action of anthelmintics inhibiting in vitro trehalose-6-phosphate phosphatase (TPP) of Anisakis simplex s.l. Exp Parasitol. 2018;184:46–56.
Mladineo I, Trumbić Ž, Hrabar J, et al. Efficiency of target larvicides is conditioned by ABC-mediated transport in the zoonotic nematode Anisakis pegreffii. Antimicrob Agents Chemother. 2018;62:1–14.
Ruckebusch Y. Données numériques et miscellanées. In: Physiologie pharmacologie thérapeutique animales. Paris: Maloine S.A.; 1977.
Hierro I, Valero A, Pérez P, et al. Action of different monoterpenic compounds against Anisakis simplex s.l. L3 larvae. Phytomedicine. 2004;11:77–82.
Navarro MC, Noguera MA, Romero MC, Montilla MP, González de Selgas JM, Valero A. Anisakis simplex s.l.: larvicidal activity of various monoterpenic derivatives of natural origin against L3 larvae in vitro and in vivo. Exp Parasitol. 2008;120:295–299.
Romero MC, Navarro MC, Martín-Sánchez J, Valero A. Peppermint (Mentha piperita) and albendazole against anisakiasis in an animal model. Trop Med Int Health. 2014;19:1430–1436.
Quiñones-Silva J, Sánchez-Aldehuelo R, Solorzano C, Zamorano M, Parejo-Carbonell S. Anisakiasis como diagnóstico diferencial de dolor abdominal agudo en urgencias. Rev Gastroenterol Peru. 2019;39:171–174. (Spanish).
Field-Cortazares J, Calderón-Campos R. Intoxicación por Anisakis. Bol Clin Hosp Infant Edo Son. 2009;26:43–47.
Fraj-Lázaro J, Remacha-Tomey B, Colás-Sanz C, Ortega-Fernández de Retana A, Lezaun-Alfonso A. Anisakis, anisakiasis and IgE-mediated immunity to Anisakis simplex. J Investig Allergol Clin Immunol. 1998;8:61–63.
Castán B, Borda F, Iñarrairaegui M, Pastor G, Vila J, Zozaya JM. Digestive anisakiasis: clinical manifestations and diagnosis according to localization. Rev Esp Enferm Dig. 2002;94:463–742.
Pinto LC, Mesquita FP, Soares BM, et al. Mebendazole induces apoptosis via C-MYC inactivation in malignant ascites cell line (AGP01). Toxicol Vitr. 2019;60:305–312.
Nawa Y, Hatz C, Blum J. Sushi delights and parasites: the risk of fishborne and foodborne parasitic zoonoses in Asia. Clin Infect Dis. 2005;41:1297–1303.
Pacios E, Arias-Díaz J, Zuloaga J, Gonzalez-Armengol J, Villarroel P, Balibrea JL. Albendazole for the treatment of anisakiasis ileus. Clin Infect Dis. 2005;41:1825–1826.
Filauro M, Rollandi GA, Cassola G, et al. Gastrointestinal bleeding due to suspected anisakiasis: challenging differential diagnosis for a rare disease. Updat Surg. 2011;63:213–217.
Pontone S, Leonetti G, Guaitoli E, et al. Should the host reaction to anisakiasis influence the treatment? Different clinical presentations in two cases. Rev Esp Enferm Dig. 2012;104:607–610.
Baptista-Fernandes T, Rodrigues M, Castro I, et al. Human gastric hyperinfection by Anisakis simplex: a severe and unusual presentation and a brief review. Int J Infect Dis. 2017;64:38–41.
Romero MC, Valero A, Navarro MC, Hierro I, Barón SD, Martín-Sánchez J. Experimental demonstration of pathogenic potential of Anisakis physeteris and Anisakis paggiae in Wistar rats. Parasitol Res. 2014;113:4377–4386.
Del Pozo V, Arrieta I, Tuñon T, et al. Immunopathogenesis of human gastrointestinal infection by Anisakis simplex. J Allergy Clin Immunol. 1999;104:637–643.
Bier JW, Raybourne RB. Anisakis simplex (Nematoda: Ascaridoides): formation of immunogenic attachment caps in pigs. Proc Helminthol Soc Wash. 1988;55:91–94.
Iglesias R. La anisakiosis y su diagnóstico [Dissertation]. Santiago de Compostela: University of Santiago de Compostela; 1998.
Bušelić I, Trumbić Ž, Hrabar J, Vrbatović A, Bočina I, Mladineo I. Molecular and cellular response to experimental Anisakis pegreffii (nematoda, anisakidae) third-stage larval infection in rats. Front Immunol. 2018;9:2055.
Kang DB, Park WC, Lee JK. Chronic gastric anisakiasis provoking a bleeding gastric ulcer. Ann Surg Treat Res. 2014;86:270–273.
Hollingworth S, Duncan E, Martin J. Marked increase in proton pump inhibitors use in Australia. Pharmacoepidemiol Drug Saf. 2010;19:1019–1024.
Ministerio de Sanidad SS e I. Subgroups ATC and active ingredients of greater consumption in the National Health System in 2010. Inf Ter del Sist Nac Salud. 2011;35:124–128. (Spanish).
Acknowledgments
The results shown in this article are part of the doctoral thesis of Magdalena Gómez-Mateos Pérez.
Funding
This work was supported by the Research Groups grant from the Junta de Andalucía, Spain [BIO-243] and a Grant to M. Gómez-Mateos from CACOF (Andalucía).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This study was approved by the ethics committee of University of Granada (approval CEE 294-2007). All procedures performed in studies comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gómez-Mateos, M., Arrebola, F., Navarro, M.C. et al. Acute Anisakiasis: Pharmacological Evaluation of Various Drugs in an Animal Model. Dig Dis Sci 66, 105–113 (2021). https://doi.org/10.1007/s10620-020-06144-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06144-2