Abstract
Objective
This study was conducted in order to assess the diagnostic accuracy of LI-RADS v2018 ancillary features (AFs) favoring malignancy applied to LR-3 and LR-4 observations on gadoxetate-enhanced MRI.
Methods
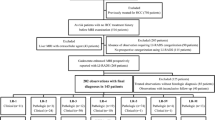
In this retrospective dual-institution study, we included consecutive patients at high risk for hepatocellular carcinoma (HCC) imaged with gadoxetate disodium-enhanced MRI between 2009 and 2014 fulfilling the following criteria: (i) at least one LR-3 or LR-4 observation ≥ 10 mm; (ii) nonrim arterial phase hyperenhancement; and (iii) confirmation of benignity or malignancy by pathology or imaging follow-up. We compared the distribution of AFs between HCCs and benign observations and the diagnostic performance for the diagnosis of HCC using univariate and multivariate analyses. Significance was set at p value < 0.05.
Results
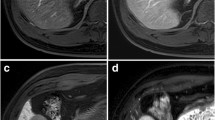
Two hundred five observations were selected in 155 patients (108 M, 47 F) including 167 (81.5%) LR-3 and 38 (18.5%) LR-4. There were 126 (61.5%) HCCs and 79 (28.5%) benign lesions. A significantly larger number of AFs favoring malignancy were found in LR-3 and LR-4 lesions that progressed to HCC compared to benign lesions (p < 0.001 and p = 0.003, respectively). The most common AFs favoring malignancy in HCCs were hepatobiliary phase (HBP) hypointensity (p < 0.001), transitional phase hypointensity (p < 0.001), and mild–moderate T2 hyperintensity (p < 0.001). Sensitivity and specificity of AFs for the diagnosis of HCC ranged 0.8–76.2% and 86.1–100%, respectively. HBP hypointensity yielded the highest sensitivity but also the lowest specificity and was the only AF remaining independently associated with the diagnosis of HCC at multivariate logistic regression analysis (OR 14.83, 95% CI 5.81–42.76, p < 0.001).
Conclusions
Among all AFs, HBP hypointensity yields the highest sensitivity for the diagnosis of HCC.
Key Points
• LR-3 and LR-4 observations diagnosed as HCC have a significantly higher number of ancillary features favoring malignancy compared to observations proven to be benign.
• The presence of three or more ancillary features favoring malignancy has a high specificity (96.2%) for the diagnosis of HCC.
• Among all ancillary features favoring malignancy, hepatobiliary phase hypointensity yields the highest sensitivity, but also the lowest specificity for the diagnosis of HCC.






Similar content being viewed by others
Abbreviations
- 3D:
-
Three-dimensional
- AFs:
-
Ancillary features
- AP:
-
Arterial phase
- APHE:
-
Arterial phase hyperenhancement
- AUC:
-
Area-under-the-curve
- DWI:
-
Diffusion-weighted images
- FSE:
-
Fast spin echo
- GRE:
-
Gradient-recalled echo
- HBP:
-
Hepatobiliary phase
- HCC:
-
Hepatocellular carcinoma
- IQR:
-
Interquartile range
- LI-RADS:
-
Liver Imaging Reporting and Data System
- MRI:
-
Magnetic resonance imaging
- NPV:
-
Negative predictive values
- OATP:
-
Organic anion transporting polypeptide
- PPV:
-
Positive predictive value
- PVP:
-
Portal venous phase
- ROC:
-
Receiver operating characteristic
- TP:
-
Transitional phase
- VIBE:
-
Volumetric interpolated breath-hold examination
References
American College of Radiology. CT/MRI Liver imaging reporting and data system v2018 core. https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CT-MRI-LI-RADS-v2018. Accessed June 2019
Chernyak V, Fowler KJ, Kamaya A et al (2018) Liver Imaging Reporting and Data System (LI-RADS) version 2018: imaging of hepatocellular carcinoma in at-risk patients. Radiology 289:816–830
Fowler KJ, Tang A, Santillan C et al (2018) Interreader reliability of LI-RADS version 2014 algorithm and imaging features for diagnosis of hepatocellular carcinoma: a large international multireader study. Radiology 286:173–185
Marrero JA, Kulik LM, Sirlin C et al (2018) Diagnosis, staging and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 68:723–750
Tang A, Bashir MR, Corwin MT et al (2018) Evidence supporting LI-RADS major features for CT- and MR imaging-based diagnosis of hepatocellular carcinoma: a systematic review. Radiology 286:29–48
Chernyak V, Tang A, Flusberg M et al (2018) LI-RADS® ancillary features on CT and MRI. Abdom Radiol (NY) 43:82–100
Cerny M, Bergeron C, Billiard JS et al (2018) LI-RADS for MR imaging diagnosis of hepatocellular carcinoma: performance of major and ancillary features. Radiology 288:118–128
Alhasan A, Cerny M, Olivié D et al (2019) LI-RADS for CT diagnosis of hepatocellular carcinoma: performance of major and ancillary features. Abdom Radiol (NY) 44:517–528
Min JH, Kim JM, Kim YK et al (2018) Prospective intraindividual comparison of magnetic resonance imaging with gadoxetic acid and extracellular contrast for diagnosis of hepatocellular carcinomas using the liver imaging reporting and data system. Hepatology 68:2254–2266
Granata V, Fusco R, Avallone A et al (2017) Critical analysis of the major and ancillary imaging features of LI-RADS on 127 proven HCCs evaluated with functional and morphological MRI: lights and shadows. Oncotarget 8:51224–51237
Ronot M, Fouque O, Esvan M, Lebigot J, Aubé C, Vilgrain V (2018) Comparison of the accuracy of AASLD and LI-RADS criteria for the non-invasive diagnosis of HCC smaller than 3 cm. J Hepatol 68:715–723
Choi SH, Byun JH, Kim SY et al (2016) Liver imaging reporting and data system v2014 with gadoxetate disodium-enhanced magnetic resonance imaging: validation of LI-RADS category 4 and 5 criteria. Invest Radiol 51:483–490
Joo I, Lee JM, Lee DH, Ahn SJ, Lee ES, Han JK (2017) Liver imaging reporting and data system v2014 categorization of hepatocellular carcinoma on gadoxetic acid-enhanced MRI: comparison with multiphasic multidetector computed tomography. J Magn Reson Imaging 45:731–740
Ludwig DR, Fraum TJ, Cannella R et al (2019) Hepatocellular carcinoma (HCC) versus non-HCC: accuracy and reliability of Liver Imaging Reporting and Data System v2018. Abdom Radiol (NY) 44:2116–2132
Cerny M, Chernyak V, Olivié D et al (2018) LI-RADS version 2018 ancillary features at MRI. Radiographics 38:1973–2001
Vernuccio F, Cannella R, Meyer M et al (2019) LI-RADS: diagnostic performance of hepatobiliary phase hypointensity and major imaging features of LR-3 and LR-4 lesions measuring 10-19 mm with arterial phase hyperenhancement. AJR Am J Roentgenol 213:W57–W65
Joo I, Lee JM, Lee DH, Jeon JH, Han JK (2019) Retrospective validation of a new diagnostic criterion for hepatocellular carcinoma on gadoxetic acid-enhanced MRI: can hypointensity on the hepatobiliary phase be used as an alternative to washout with the aid of ancillary features? Eur Radiol 29:1724–1732
Kim DH, Choi SH, Kim SY, Kim MJ, Lee SS, Byun JH (2019) Gadoxetic acid-enhanced MRI of hepatocellular carcinoma: value of washout in transitional and hepatobiliary phases. Radiology 291:651–657
Kim SS, Kim SH, Song KD, Choi SY, Heo NH (2019) Value of gadoxetic acid-enhanced MRI and diffusion-weighted imaging in the differentiation of hypervascular hyperplastic nodule from small (<3 cm) hypervascular hepatocellular carcinoma in patients with alcoholic liver cirrhosis: a retrospective case-control study. J Magn Reson Imaging. https://doi.org/10.1002/jmri.26768
Cho YK, Kim JW, Kim MY, Cho HJ (2018) Non-hypervascular hypointense nodules on hepatocyte phase gadoxetic acid-enhanced MR images: transformation of MR hepatobiliary hypointense nodules into hypervascular hepatocellular carcinomas. Gut Liver 12:79–85
Briani C, Di Pietropaolo M, Marignani M et al (2018) Non-hypervascular hypointense nodules at gadoxetic acid MRI: hepatocellular carcinoma risk assessment with emphasis on the role of diffusion-weighted imaging. J Gastrointest Cancer 49:302–310
Cha DI, Jang KM, Kim SH, Kang TW, Song KD (2017) Liver Imaging Reporting and Data System on CT and gadoxetic acid-enhanced MRI with diffusion-weighted imaging. Eur Radiol 27:4394–4405
Kamath A, Roudenko A, Hecht E et al (2019) CT/MR LI-RADS 2018: clinical implications and management recommendations. Abdom Radiol (NY) 44:1306–1322
Kim YY, An C, Kim S, Kim MJ (2018) Diagnostic accuracy of prospective application of the Liver Imaging Reporting and Data System (LI-RADS) in gadoxetate-enhanced MRI. Eur Radiol 28:2038–2046
Hong CW, Park CC, Mamidipalli A et al (2019) Longitudinal evolution of CT and MRI LI-RADS v2014 category 1, 2, 3, and 4 observations. Eur Radiol. https://doi.org/10.1007/s00330-019-06058-2
Choi SH, Byun JH, Lim YS et al (2018) Liver Imaging Reporting and Data System: patient outcomes for category 4 and 5 nodules. Radiology 287:515–524
Choi JY, Cho HC, Sun M, Kim HC, Sirlin CB (2013) Indeterminate observations (liver imaging reporting and data system category 3) on MRI in the cirrhotic liver: fate and clinical implications. AJR Am J Roentgenol 201:993–1001
Tanabe M, Kanki A, Wolfson T et al (2016) Imaging outcomes of liver imaging reporting and data system version 2014 category 2, 3, and 4 observations detected at CT and MR imaging. Radiology 281:129–139
Burke LM, Sofue K, Alagiyawanna M et al (2016) Natural history of liver imaging reporting and data system category 4 nodules in MRI. Abdom Radiol (NY) 41:1758–1766
van der Pol CB, Lim CS, Sirlin CB et al (2019) Accuracy of the Liver Imaging Reporting and Data System in computed tomography and magnetic resonance image analysis of hepatocellular carcinoma or overall malignancy—a systematic review. Gastroenterology 156:976–986
Sofue K, Burke LMB, Nilmini V et al (2017) Liver imaging reporting and data system category 4 observations in MRI: risk factors predicting upgrade to category 5. J Magn Reson Imaging 46:783–792
Song JS, Choi EJ, Hwang SB, Hwang HP, Choi H (2019) LI-RADS v2014 categorization of hepatocellular carcinoma: intraindividual comparison between gadopentetate dimeglumine-enhanced MRI and gadoxetic acid-enhanced MRI. Eur Radiol 29:401–410
Kierans AS, Makkar J, Guniganti P et al (2018) Validation of Liver Imaging Reporting and Data System 2017 (LI-RADS) criteria for imaging diagnosis of hepatocellular carcinoma. J Magn Reson Imaging 49:e205–e215
Fraum TJ, Tsai R, Rohe E, Ludwig DR et al (2018) Differentiation of hepatocellular carcinoma from other hepatic malignancies in patients at risk: diagnostic performance of the Liver Imaging Reporting and Data System Version 2014. Radiology 286:158–172
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Alessandro Furlan.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: Daniele Marin: research support from Siemens Healthineers; and Alessandro Furlan: royalties from Elsevier/Amirsys and consultant for Bracco.
Statistics and biometry
Two of the authors (Hersh Sagreiya and Kingshuk Roy Choudhury) have significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
We would like to specify that 104/155 patients were included in a previous study (title: LI-RADS: diagnostic performance of hepatobiliary phase hypointensity and major imaging features of LR-3 and LR-4 lesions measuring 10–19 mm with arterial phase hyperenhancement) where we reported the diagnostic performance of the HBP hypointensity and major features in LR-3 and LR-4 observations with APHE measuring 10–19 mm and classified according to LI-RADS v2017. Conversely, in this study, we aim at investigating the diagnostic performance of all AFs favoring malignancy, not only HBP hypointensity, for the diagnosis of HCC using the LI-RADS v2018.
Methodology
• Retrospective
• Diagnostic or prognostic study
• Multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 25 kb)
Rights and permissions
About this article
Cite this article
Cannella, R., Vernuccio, F., Sagreiya, H. et al. Liver Imaging Reporting and Data System (LI-RADS) v2018: diagnostic value of ancillary features favoring malignancy in hypervascular observations ≥ 10 mm at intermediate (LR-3) and high probability (LR-4) for hepatocellular carcinoma. Eur Radiol 30, 3770–3781 (2020). https://doi.org/10.1007/s00330-020-06698-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-06698-9