Abstract
The present research paper reports the convenient synthesis, successful characterization, in vitro antibacterial, antifungal, antioxidant potency and biocompatibility of N-acyl-morpholine-4-carbothioamides (5a–5j). The biocompatible derivatives were found to be highly active against the tested bacterial and fungal strains. Moreover, some of the screened N-acyl-morpholine-4-carbothioamides exhibited excellent antioxidant potential. Docking simulation provided additional information about possibilities of their inhibitory potential against RNA. It has been predicted by in silico investigation of the binding pattern that compounds 5a and 5j can serve as the potential surrogate for design of novel and potent antibacterial agents. The results for the in vitro bioassays were promising with the identification of compounds 5a and 5j as the lead and selective candidate for RNA inhibition. Results of the docking computations further ascertained the inhibitory potential of compound 5a. Based on the in silico studies, it can be suggested that compounds 5a and 5j can serve as a structural model for the design of antibacterial agents with better inhibitory potential.
Graphic abstract
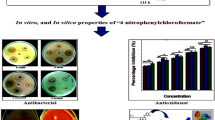
Binding mode of compound 5j inside the active site of RNA in 3D space. 5j displayed highest antibacterial potential than the reference drug ampicillin with ZOI 10.50 mm against Staphylococcus aureus. 5j also displayed highest antifungal potential than the reference drug amphotericin B with ZOI 18.20 mm against Fusarium solani.






Similar content being viewed by others
References
Nikaido H (2009) Multidrug resistance in bacteria. Ann Rev Biochem 78:119–146
Ascioglu S, Rex JH, De Pauw B, Bennett JE, Bille J, Crokaert F, Fiere D (2002) Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 34(1):7–14
Moneer AA, Abouzid KAM, Said MM (2002) Synthesis of certain thiopyrimidine derivatives as antimicrobial agents. Az J Pharm Sci 30:150–160
Suhas R, Chandrashekar S, Gowda DC (2012) Synthesis of uriedo and thiouriedo derivatives of peptide conjugated heterocycles—a new class of promising antimicrobials. Eur J Med Chem 48:179–191
Suresha GP, Suhas R, Kapfo W, Gowda DC (2011) Urea/thiourea derivatives of quinazolinone–lysine conjugates: synthesis and structure–activity relationships of a new series of antimicrobials. Eur J Med Chem 46(6):2530–2540
Tuncel ST, Gunal SE, Ekizoglu M, Kelekci NG, Erdem SS, Bulak E, Dogan I (2019) Thioureas and their cyclized derivatives: synthesis, conformational analysis and antimicrobial evaluation. J Mol Struct 1179:40–56
Bielenica A, Stefańska J, Stępień K, Napiórkowska A, Augustynowicz-Kopeć E, Sanna G, Struga M (2015) Synthesis, cytotoxicity and antimicrobial activity of thiourea derivatives incorporating 3-(trifluoromethyl) phenyl moiety. Eur J Med Chem 101:111–125
Saeed A, Mustafa MN, Zain-ul-Abideen M, Shabir G, Erben MF, Flörke U (2019) Current developments in chemistry, coordination, structure and biological aspects of 1-(acyl/aroyl)-3-(substituted) thioureas: advances Continue…. J Sulfur Chem 40(3):312–350
Pingaew R, Prachayasittikul V, Anuwongcharoen N, Prachayasittikul S, Ruchirawat S, Prachayasittikul V (2018) Synthesis and molecular docking of N,N′-disubstituted thiourea derivatives as novel aromatase inhibitors. Bioorg Chem 79:171–178
Pérez H, O’Reilly B, Plutín AM, Martínez R, Durán R, Collado IG, Mascarenhas YP (2011) Synthesis, characterization, and crystal structure of Ni(II) and Cu(II) complexes with N-furoyl-N′,N′-diethylthiourea: antifungal activity. J Coord Chem 64(16):2890–2898
Saad FA, Buurma NJ, Amoroso AJ, Knight JC, Kariuki BM (2012) Co-ordination behaviour of a novel bisthiourea tripodal ligand: structural, spectroscopic and electrochemical properties of a series of transition metal complexes. Dalton Trans 41(15):4608–4617
Selvakumaran N, Ng SW, Tiekink ER, Karvembu R (2011) Versatile coordination behavior of N,N-di(alkyl/aryl)-N′-benzoylthiourea ligands: synthesis, crystal structure and cytotoxicity of palladium(II) complexes. Inorg Chim Acta 376(1):278–284
Phuong T, Khac-Minh T, Van Ha NT, Phuong HTN (2004) Synthesis and antifungal activities of phenylenedithioureas. Bioorg Med Chem Lett 14(3):653–656
Zhong Z, Xing R, Liu S, Wang L, Cai S, Li P (2008) Synthesis of acyl thiourea derivatives of chitosan and their antimicrobial activities in vitro. Carbohyd Res 343(3):566–570
Saeed A, Flörke U, Erben MF (2014) A review on the chemistry, coordination, structure and biological properties of 1-(acyl/aroyl)-3-(substituted) thioureas. J Sulfur Chem 35(3):318–355
Shah DR, Lakum HP, Chikhalia KH (2015) Synthesis and in vitro antimicrobial evaluation of piperazine substituted quinazoline-based thiourea/thiazolidinone/chalcone hybrids. Russ J Bioorg Chem 41(2):209–222
Keche AP, Kamble VM (2014) Synthesis and anti-inflammatory and antimicrobial activities of some novel 2-methylquinazolin-4 (3H)-one derivatives bearing urea, thiourea and sulphonamide functionalities. Arab J Chem 7(12):1522–1531
Abbas SY, El-Sharief MAS, Basyouni WM, Fakhr IM, El-Gammal EW (2013) Thiourea derivatives incorporating a hippuric acid moiety: synthesis and evaluation of antibacterial and antifungal activities. Eur J Med Chem 64:111–120
Erşen D, Ülger M, Mangelinckx S, Gemili M, Şahin E, Nural Y (2017) Synthesis and anti (myco) bacterial activity of novel 5,5-diphenylpyrrolidine N-aroylthiourea derivatives and a functionalized hexahydro-1H-pyrrolo[1,2-c]imidazole. Med Chem Res 26(9):2152–2160
Cui P, Li X, Zhu M, Wang B, Liu J, Chen H (2017) Design, synthesis and antibacterial activities of thiouracil derivatives containing acyl thiourea as SecA inhibitors. Bioorg Med Chem Lett 27(10):2234–2237
Uckun FM, Pendergrass S, Maher D, Zhu D, Tuel-Ahlgren L, Mao C, Venkatachalam TK (1999) N′-[2-(2-Thiophene) ethyl]-N′-[2-(5-bromopyridyl)] thiourea as a potent inhibitor of NNI-resistant and multidrug-resistant human immunodeficiency virus-1. Bioorg Med Chem Lett 9(24):3411–3416
Qasim M, Abideen Z, Adnan MY, Gulzar S, Gul B, Rasheed M, Khan MA (2017) Antioxidant properties, phenolic composition, bioactive compounds and nutritive value of medicinal halophytes commonly used as herbal teas. South Afr J Bot 110:240–250
Shon MY, Kim TH, Sung NJ (2003) Antioxidants and free radical scavenging activity of Phellinus baumii (Phellinus of Hymenochaetaceae) extracts. Food Chem 82(4):593–597
Zia-Ul-Haq M, Shahid SA, Ahmad S, Qayum M, Khan I (2012) Antioxidant potential of various parts of Ferula assafoetida L. J Med Plants Res 6(16):3254–3258
Evans BC, Nelson CE, Shann SY, Beavers KR, Kim AJ, Li H, Duvall CL (2013) Ex vivo red blood cell hemolysis assay for the evaluation of pH-responsive endosomolytic agents for cytosolic delivery of biomacromolecular drugs. J Vis Exp 73:e50166
Amin K, Dannenfelser RM (2006) In vitro hemolysis: guidance for the pharmaceutical scientist. J Pharma Sci 95(6):1173–1176
Pisano MB, Kumar A, Medda R, Gatto G, Pal R, Fais A, Era B, Cosentino S, Uriarte E, Santana L, Pintus F, Matos MJ (2019) Antibacterial activity and molecular docking studies of a selected series of hydroxy-3-arylcoumarins. Molecules 24(15):2815
Mendoza-Figueroa HL, Serrano-Alva MT, Aparicio-Ozores G, Martínez-Gudiño G, Suárez-Castillo OR, Pérez-Rojas NA, Morales-Ríos MS (2018) Synthesis, antimicrobial activity, and molecular docking study of fluorine-substituted indole-based imidazolines. Med Chem Res 27:1624
Liu JS, Deng LJ, Tian HY, Ruan ZX, Cao HH, Ye WC, Yu ZL (2019) Anti-tumor effects and 3D-quantitative structure–activity relationship analysis of bufadienolides from toad venom. Fitoterapia 134:362–371
Ruan ZX, Huangfu DS, Sun PH, Chen WM (2013) Molecular modeling studies on 3,4-dihydroquinazolines as trypanothione reductase inhibitors using 3D-QSAR and docking approaches. Med Chem Res 22(8):3541–3555
Ruan ZX, Huangfu DS, Xu XJ, Sun PH, Chen WM (2013) 3D-QSAR and molecular docking for the discovery of ketolide derivatives. Exp Opin Drug Disc 8(4):427–444
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (1997) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 23(1–3):3–25
Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD (2002) Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem 45(12):2615–2623
Jamil M, ul Haq I, Mirza B, Qayyum M (2012) Isolation of antibacterial compounds from Quercus dilatata L. through bioassay guided fractionation. Ann Clin Microb Antimicrob 11(1):11
Ahmed M, Fatima H, Qasim M, Gul B (2017) Polarity directed optimization of phytochemical and in vitro biological potential of an indigenous folklore: Quercus dilatata Lindl. ex Royle. BMC Comp Altern Med 17(1):386
Brand-Williams W, Cuvelier ME, Berset CLWT (1995) Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 28(1):25–30
Umamaheswari M, Chatterjee TK (2008) In vitro antioxidant activities of the fractions of Coccinia grandis L. leaf extract. Afr J Trad Comp Altern Med 5(1):61–73
Siddhuraju P, Mohan PS, Becker K (2002) Studies on the antioxidant activity of Indian Laburnum (Cassia fistula L.): a preliminary assessment of crude extracts from stem bark, leaves, flowers and fruit pulp. Food Chem 79(1):61–67
Molecular Operating Environment, 2015.08 (2015) Chemical Computing Group Inc., 1010 Sherbooke St. West, Suite 910, Montreal, QC, Canada, H3A 2R7
Halgren TA, Nachbar RB (1996) Merck molecular force field. IV. Conformational energies and geometries for MMFF94. J Comput Chem 17(5–6):587–615
Vicens Q, Westhof E (2001) Crystal structure of paromomycin docked into the eubacterial ribosomal decoding A site. Structure 9(8):647–658
Menozzi G, Merello L, Fossa P, Schenone S, Ranise A, Mosti L, La Colla P (2004) Synthesis, antimicrobial activity and molecular modeling studies of halogenated 4-[1H-imidazol-1-yl(phenyl)methyl]-1,5-diphenyl-1H-pyrazoles. Bioorg Med Chem 12(20):5465–5483
Acknowledgements
The author Mr. Hamid Aziz is highly grateful to the Higher Education Commission (HEC), Pakistan, for providing indigenous scholarship as the financial support for the research work performed.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aziz, H., Saeed, A., Khan, M.A. et al. Synthesis, characterization, antimicrobial, antioxidant and computational evaluation of N-acyl-morpholine-4-carbothioamides. Mol Divers 25, 763–776 (2021). https://doi.org/10.1007/s11030-020-10054-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-020-10054-w