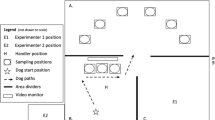
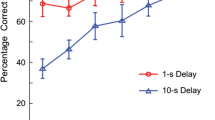
Abstract
Working memory is essential for organisms to solve problems related to their survival and to adapt to changes in their environment. Researchers sought to create a non-human model of working memory that could be used to better understand its predictive value and underlying brain function. Several of these studies were conducted using the odor span task (OST) with rodents, and here, we present the first OST with domestic dogs (n = 6). The OST is an incrementing non-match-to-sample task in which dogs were presented with both a session novel (S +) and a previously encountered (S −) odor on each trial. A response to the session novel odor was always reinforced. Upon meeting training criterion on sessions with 24 trials or odors to remember, the dogs were tested on the OST with up to 72 odors to remember in the session. All dogs learned the OST and displayed accurate performance (≥ 79%) for the largest set size of 72 odors. In an analysis focused on the effect of intervening odors (i.e., the number of trials since the S − was last encountered), dogs demonstrated above-chance performance for up to eight intervening odors. The implications of these findings are discussed in the context of dog working memory for odors.




Similar content being viewed by others
References
Adams B, Chan A, Callahan H, Milgram NW (2000) The canine as a model of human cognitive aging: recent developments. Progr Neuro-Psychopharmacol Biol Psychiatry 24(5):675–692
April LB, Bruce K, Galizio M (2013) The magic number 70 (plus or minus 20): variables determining performance in the rodent odor span task. Learn Motiv 44(3):143–158
Baddeley AD (2017) The concept of working memory: A view of its current state and probable future development. Exploring Working Memory Routledge, pp 99–106
Bain MJ, Hart BL, Cliff KD, Ruehl WW (2001) Predicting behavioral changes associated with age-related cognitive impairment in dogs. J Am Vet Med Assoc 218(11):1792–1795
Basile BM, Hampton RR (2013) Dissociation of active working memory and active working memory and passive recognition in rhesus monkeys. Cognition 126(3):391–396
Bensky MK, Gosling SD, Sinn DL (2013) The world from a dog’s point of view: a review and synthesis of dog cognition research. Adv Study Behav 45:209–406
Brady RJ, Hampton RR (2018) Nonverbal working memory for novel images in rhesus monkeys. Curr Biol 28(24):3903–3910
Bratch A, Kann S, Cain JA, Wu JE, Rivera-Reyes N, Dalecki S, Arman D, Dunn A, Cooper S, Corbin HE, Doyle AR, Pizzo MJ, Smith AE, Crystal J (2016) Working memory systems in the rat. Curr Biol 26(3):351–355
Bray EE, MacLean EL, Hare BA (2015) Increasing arousal enhances inhibitory control in calm but not excitable dogs. Anim Cogn 18(6):1317–1329
Chan AD, Nippak P, Murphey H, Ikeda-Douglas CJ, Muggenburg B, Head E, Cotman CW, Milgram NW (2002) Visuospatial impairments in aged canines (Canis familiaris): the role of cognitive-behavioral flexibility. Behav Neurosci 116(3):443
Chase WG, Simon HA (1973) Perception in chess. Cogn Psychol 4(1):55–81
Cobb M, Branson N, McGreevy P, Lill A, Bennett P (2015) The advent of canine performance science: offering a sustainable future for working dogs. Behav Proc 110:96–104
Conway AR, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW (2005) Working memory span tasks: A methodological review and user’s guide. Psychon Bull Rev 12(5):769–786
Cowan N (2017) The many faces of working memory and short-term storage. Psychon Bull Rev 24(4):1158–1170
Daneman M, Carpenter PA (1980) Individual differences in working memory and reading. J Verbal Learn Verbal Behav 19(4):450–466
Dudchenko PA (2004) An overview of the tasks used to test working memory in rodents. Neurosci Biobehav Rev 28(7):699–709
Dudchenko PA, Wood ER, Eichenbaum H (2000) Neurotoxic hippocampal lesions have no effect on odor span and little effect on odor recognition memory but produce significant impairments on spatial span, recognition, and alternation. J Neurosci 20(8):2964–2977
Engle RW, Kane MJ (2004) Executive attention, working memory capacity, and a two-factor theory of cognitive control. Psychol Learn Mot 44:145–200
Galizio M, April B, Deal M, Hawkey A, Panoz-Brown D, Prichard A, Bruce K (2016) Behavioral pharmacology of the odor span task: Effects of flunitrazepam, ketamine, methamphetamine and methylphenidate. J Exp Anal Behav 106(3):173–194
Hall NJ, Smith DW, Wynne CD (2013) Training domestic dogs (Canis lupus familiaris) on a novel discrete trials odor-detection task. Learn Motiv 44(4):218–228
Head E, Rofina J, Zicker S (2008) Oxidative stress, aging, and central nervous system disease in the canine model of human brain aging. Vet Clin 38(1):167–178
Honig WK (1978) Studies of working memory in the pigeon. Cognit Processes Anim Behav 211–248
Kirchner WK (1958) Age differences in short-term retention of rapidly changing information. J Exp Psychol 55(4):352
IleLépine R, Parrouillet P, Camos V (2005) What makes working memory spans so predictive of high-level cognition. Psychon Bull Rev 12(1):165–170
Lind J, Enquist M, Ghirlanda S (2015) Animal memory: A review of delayed matching-to-sample data. Behav Proc 117:52–58
Ma WJ, Husain M, Bays P (2014) Changing concepts in working memory. Nat Neurosci 17:347–356
Maejima M, Inoue-Murayama M, Tonosaki K, Matsuura N, Kato S, Saito Y, Weiss A, Murayama Y, Ito SI (2007) Traits and genotypes may predict the successful training of drug detection dogs. Appl Anim Behav Sci 107(3):287–298
MacLean EL, Hare B (2018) Enhanced selection of assistance and explosive detection dogs using cognitive measures. Front Vet Sci 5:236
Milgram NW, Head E, Weiner E, Thomas E (1994) Cognitive functions and aging in the dog: acquisition of nonspatial visual tasks. Behav Neurosci 108(1):57
Miller GA (1956) The magical number seven, plus or minus two: Some limits on our capacity for processing information. Psychol Rev 101(2):343–352
Olton DS, Samuelson RJ (1976) Remembrance of places passed: spatial memory in rats. J Exp Psychol 2(2):97
PañozBrown D, Corbin H, Dalecki S, Gentry M, Brotheridge S, Sluka C, Wu J, Crystal J (2016) Rats remember items in context using episodic memory. Current Biol 26(20):2821–2826
Polgár Z, Kinnunen M, Újváry D, Miklósi Á, Gácsi M (2016) A test of canine olfactory capacity: comparing various dog breeds and wolves in a natural detection task. PLoS ONE 11(5):e0154087
Porritt F, Shapiro M, Waggoner P, Mitchell E, Thomson T, Nicklin S, Kacelnik A (2015) Performance decline by search dogs in repetitive tasks, and mitigation strategies. Appl Anim Behav Sci 166:112–122
Redick TS, Lindsey DR (2013) Complex span and n-back measures ofworking memory: a meta-analysis. Psychon Bull Rev 20(6):1102–1113
Salvin HE, McGreevy PD, Sachdev PS, Valenzuela MJ (2011) Growing old gracefully—Behavioral changes associated with “successful aging” in the dog, Canis familiaris. J Vet Behav 6(6):313–320
Scharfen J, Jansen K, Holling H (2018) Retest effects in working memory capacity tests: A meta-analysis. Psychon Bull Rev 25:2175–2199
Studzinski CM, Christie LA, Araujo JA, Burnham WM, Head E, Cotman CW, Milgram NW (2006) Visuospatial function in the beagle dog: an early marker of cognitive decline in a model of human aging and dementia. Neurobiol Learn Memory 86(2):197–204
Tapp PD, Siwak CT, Estrada J, Head E, Muggenburg BA, Cotman CW, Milgram NW (2003) Size and reversal learning in the beagle dog as a measure of executive function and inhibitory control in aging. Learn Mem 10(1):64–73
Troisi CA, Mills DS, Wilkinson A, Zulch HE (2019) Behavioral and cognitive factors that affect the success of scent detection dogs. Comp Cognit Behav Rev 14:51–67
Turner ML, Engle RW (1989) Is working memory capacity task dependent? J Mem Lang 28(2):127–154
Yonelinas AP (2002) The nature of recollection and familiarity: A review of years of 30 years of research. J Mem Lang 46(3):441–517
Wright AA (2012) Memory processing. In: Zentall TR, Wasserman EA (eds) The Oxford Handbook of Comparative Cognition. Oxford University Press, New York, pp 239–260
Wright AA, Katz JS, Ma WJ (2012) How to be proactive about interference: Lessons from animal memory. Psychol Sci 23(5):453–458
Zanghi BM, Araujo J, Milgram NW (2015) Cognitive domains in the dog: independence of working memory from object learning, selective attention, and motor learning. Anim Cognit 18(3):789–800
Acknowledgements
The authors thank Lucia Lazarowski and Lily Strassberg for assistance with data collection and design. We are grateful to Canine Performance Sciences administrative, veterinary, and training staff for accommodating testing of the dogs and providing logistical support. This research was supported in part by a grant from the Association of Professional Dog Trainers Foundation to the first author.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
This research complied with the current laws of the United States of America and was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at Auburn University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Krichbaum, S., Rogers, B., Cox, E. et al. Odor span task in dogs (Canis familiaris). Anim Cogn 23, 571–580 (2020). https://doi.org/10.1007/s10071-020-01362-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-020-01362-7