Abstract
Purpose
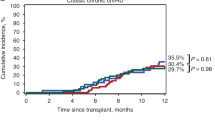
On August 2, 2017, the Food and Drug Administration approved ibrutinib (IMBRUVICA) for the treatment of patients with chronic graft versus host disease (cGVHD) after the failure of one or more lines of systemic therapy. The approval was based on results from a single-arm, multicenter trial that enrolled patients with refractory cGVHD. This paper describes the FDA review of patient-reported outcomes (PRO) data from Study PCYC-1129-CA and the decision to incorporate descriptive PRO data in the FDA label to support the primary clinician-reported outcome results.
Methods
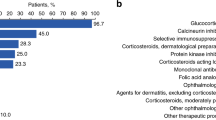
In this trial, the Lee Chronic GVHD Symptom Scale (LSS) was used to capture patient-reported symptom bother. The 42 patients who received treatment were included in the analysis and completed the PRO tool. Post hoc descriptive analyses were conducted to further understand the measurement properties of the LSS.
Results
The analysis submitted to FDA reported that 18 patients had a ≥ 7-point improvement on the LSS overall summary score at any point during the assessment period. For 10 patients, the ≥ 7-point improvement was sustained for ≥ 2 consecutive PRO assessments. An assessment of the responder threshold suggested the threshold submitted to the FDA was reasonable and in line with clinical findings.
Conclusions
Overall, study PCYC-1129-CA demonstrated favorable clinician-reported cGVHD efficacy results that were complemented by results from PRO data, supporting the FDA’s positive benefit-risk assessment leading to regular approval. Limitations included the single-arm trial design, responder definition, and instrument shortcomings. These limitations were thoroughly explored through additional FDA post hoc analyses.

Similar content being viewed by others
References
Flowers, M. E. D., & Martin, P. J. (2014). How we treat chronic graft-versus-host disease. Blood. https://doi.org/10.1182/blood-2014-08-551994.
Martin, P. J., Counts, G. W., Appelbaum, F. R., Lee, S. J., Sanders, J. E., Deeg, H. J., et al. (2010). Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. Journal of Clinical Oncology,28(6), 1011–1016. https://doi.org/10.1200/JCO.2009.25.6693.
Wingard, J. R., Majhail, N. S., Brazauskas, R., Wang, Z., Sobocinski, K. A., Jacobsohn, D., et al. (2011). Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. Journal of Clinical Oncology,29(16), 2230–2239. https://doi.org/10.1200/JCO.2010.33.7212.
Drugs @ FDA Full Prescribing Information—Imbruvica. Retrieved January 4, 2018, from https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210563s000lbl.pdf.
FDA-NIH Biomarker Working Group. (2016). BEST (Biomarkers, EndpointS, and other Tools) Resource.
Lee, S. J., Cook, E. F., Soiffer, R., & Antin, J. H. (2002). Development and validation of a scale to measure symptoms of chronic graft-versus-host disease. Biology of Blood and Marrow Transplantation,8(8), 444–452. https://doi.org/10.1053/bbmt.2002.v8.pm12234170.
Pidala, J., Kurland, B. F., Chai, X., Vogelsang, G., Weisdorf, D. J., Pavletic, S., et al. (2011). Sensitivity of changes in chronic graft-versus-host disease activity to changes in patient-reported quality of life: Results from the Chronic Graft-versus-Host Disease Consortium. Haematologica,96(10), 1528–1535.
Inamoto, Y., Martin, P. J., Chai, X., Jagasia, M., Palmer, J., Pidala, J., et al. (2012). Clinical benefit of response in chronic graft-versus-host disease. Biology of Blood and Marrow Transplantation,18(10), 1517–1524. https://doi.org/10.1016/j.bbmt.2012.05.016.
US Department of Health and Human Services; US Food and Drug Administration; Center for Drug Evaluation and Research (CDER); Center for Biologics Evaluation and Research (CBER); Center for Devices and Radiological Health (CDRH). (2009). Guidance for industry. Patient-reported outcome measures: Use in medical product development to support labeling claims. Silver Spring, MD.
Pavletic, S. Z., Martin, P., Lee, S. J., Mitchell, S., Jacobsohn, D., Cowen, E. W., et al. (2006). Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. Response criteria working group report. Biology of Blood and Marrow Transplantation,12(3), 252–266.
Miklos, D., Cutler, C. S., Arora, M., Waller, E. K., Jagasia, M., Pusic, I., et al. (2017). Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. https://doi.org/10.1182/blood-2017-07-793786.
Lee, S. J., Wolff, D., Kitko, C., Koreth, J., Inamoto, Y., Jagasia, M., et al. (2015). Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 Response Criteria Working Group report. Biology of Blood and Marrow Transplantation,21(6), 984–999. https://doi.org/10.1016/j.bbmt.2015.02.025.
Osoba, D., Bezjak, A., Brundage, M., Zee, B., Tu, D., Pater, J., et al. (2005). Analysis and interpretation of health-related quality-of-life data from clinical trials: Basic approach of The National Cancer Institute of Canada Clinical Trials Group. European Journal of Cancer,41(2), 280–287. https://doi.org/10.1016/j.ejca.2004.10.017.
Yost, K. J., Cella, D., Chawla, A., Holmgren, E., Eton, D. T., Ayanian, J. Z., et al. (2005). Minimally important differences were estimated for the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) instrument using a combination of distribution- and anchor-based approaches. Journal of Clinical Epidemiology,58(12), 1241–1251. https://doi.org/10.1016/j.jclinepi.2005.07.008.
Osoba, D., Rodrigues, G., Myles, J., Zee, B., & Pater, J. (1998). Interpreting the significance of changes in health-related quality-of-life scores. Journal of Clinical Oncology,16(1), 139–144. https://doi.org/10.1200/JCO.1998.16.1.139.
Norman, G. R., Sloan, J. A., & Wyrwich, K. W. (2003). Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Medical Care,41(5), 582–592.
National Comprehensive Cancer Network. Hematopoietic Cell Transplantation: Pre-Transplantation Recipient Evaluation and Management of Graft-Versus-Host Disease (Version 1.2020). Retrieved November 22, 2019, from https://www.nccn.org/professionals/physician_gls/pdf/hct.pdf.
Martin, P. J., Storer, B. E., Inamoto, Y., Flowers, M. E. D., Carpenter, P. A., Pidala, J., et al. (2017). An endpoint associated with clinical benefit after initial treatment of chronic graft-versus-host disease. Blood,130(3), 360–367. https://doi.org/10.1182/blood-2017-03-775767.
Arai, S., Pidala, J., Pusic, I., Chai, X., Jaglowski, S., Khera, N., et al. (2016). A randomized phase II crossover study of imatinib or rituximab for cutaneous sclerosis after hematopoietic cell transplantation. Clinical Cancer Research,22(2), 319–327. https://doi.org/10.1158/1078-0432.CCR-15-1443.
Funding
None.
Author information
Authors and Affiliations
Contributions
BKK, TG, ADC, VK and PK designed the research, BKK and ADC conducted analyses, TW, TG, AF and PK provided oversight for the analyses, BKK, TW, VK, ADC, VB, AF and PK wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Ethical approval
IRB: This is US Government work and is a secondary analysis of data that was submitted to the Food and Drug Administration, therefore, IRB was not applicable. The trial identifier is NCT02195869.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
King-Kallimanis, B.L., Wroblewski, T., Kwitkowski, V. et al. FDA review summary of patient-reported outcome results for ibrutinib in the treatment of chronic graft versus host disease. Qual Life Res 29, 1903–1911 (2020). https://doi.org/10.1007/s11136-020-02448-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-020-02448-y