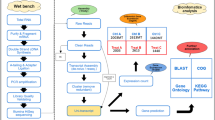
Abstract
Different responsive abilities of different types of eels (cultured, cultured feminized, and wild silver eels) during artificial maturation are recognized, and maturity status at the beginning of artificial maturation might be important. Maturity may represented by the distribution pattern of oocyte diameters. Androgens have been demonstrated to stimulate ovarian development in eels. To determine the initial status, operations were performed on eels to identify sex and to sample ovarian tissue. The recovered eels were then treated with 17α-methyltestosterone (17MT), and the responses of individual eels to 17MT were determined by the fold change in the mean oocyte diameter before and after treatment. Sampled ovarian tissues were fixed in Bouin’s solution, oocytes were isolated, and the diameter of isolated oocytes was measured. The ovarian status, determined by kernel density estimation (KDE), was presented by the probability density of measured oocyte diameters; compared with histograms, a description method, KDE, provided more subtle information on the investigated ovary. Our data indicated a correlation between the initial ovarian status (density pattern) and the consequence of treatment (change of ovary); we also argued the semelparity of the Japanese eel. Our results supported the hypothesis that the initial ovarian status is an important factor affecting artificial maturation and that androgens could ameliorate the initial status of the eel ovary.






Similar content being viewed by others
References
Arjona FJ, Vargas-Chacoff L, Martín Del Río MP, Flik G, Mancera JM, Klaren PHM (2011) Effects of cortisol and thyroid hormone on peripheral outer ring deiodination and osmoregulatory parameters in the Senegalese sole (Solea senegalensis). J Endocrinol 208:323–330
Hanel R, Stepputtis D, Bonhommeau S, Castonguay M, Schaber M, Wysujack K, Vobach M, Miller MJ (2014) Low larval abundance in the Sargasso Sea: new evidence about reduced recruitment of the Atlantic eels. Naturwissenschaften 101(12):1041–1054
Higuchi M, Mekuchi M, Hano T, Imaizumi H (2019) Trans-omics analyses revealed differences in hormonal and nutritional status between wild and cultured female Japanese eel (Anguilla japonica). PLoS One 14(5):e0209063. https://doi.org/10.1371/journal.pone.0209063
Huang YS, Chen YM, Liao PC, Lee YH, Gwo JC, Chen MC, Chang CF (2012) Testosterone improves the transition of primary oocytes in artificial maturation eels (Anguilla japonica) by altering ovarian PTEN expression. Fish Physiol Biochem 38(3):777–787. https://doi.org/10.1007/s10695-011-9560-6
Ijiri S, Kayaba T, Takeda N, Tachiki H, Adachi S, Yamauchi K (1998) Pretreatment reproductive stage and oocyte development induced by salmon pituitary homogenate in the Japanese eel Anguilla japonica. Fish Sci 64(4):531–537
Ijiri S, Tsukamoto K, Seinen Chow S, Kurogi H, Adachi S, Tanaka H (2011) Controlled reproduction in the Japanese eel (Anguilla japonica), past and present. Aquac Europe 36(2):13–17
Kagawa H, Iinuma N, Tanaka H, Ohta H, Okuzawa K (1998) Effects of rearing period in seawater on induced maturation in female Japanese eel Anguilla japonica. Fish Sci 64(1):77–82
Kazeto Y, Ijiri S, Todo T, Adachi S, Yamauchi K (2000) Molecular cloning and characterization of Japanese eel ovarian P450c17 (CYP17) cDNA. Gen Comp Endocrinol 118:123–133
Kolli R, Cummings B, Glenn T, Bringolf R, Kenneke J (2017) Epigenetic mechanism mediating the regulation of the estrogen exposure biomarker vitellogenin in zebrafish. Gordon Research Conference on Cellular and Molecular Mechanisms of Toxicity, NH, Andover, August 13–18, 2017
Krzywinski M, Altman N (2013) Error bars. Nat Methods 10:921–922
Lai XJ, Li ZQ, Xie YJ, Chen SX, Wang YL (2018) Androstenedione and 17α-methyltestosterone induce early ovary development of Anguilla japonica. Theriogenology 120:16–24
Lee SC, Lou SW (2019) Androgenic modulation in the primary ovarian growth of the Japanese eel, Anguilla japonica. Zool Stud 58:2. https://doi.org/10.6620/ZS.2019.58-02
Lin HR, Zhang ML, Zhang SM, Van Der Kraak G, Peter RE (1991) Stimulation of pituitary gonadotropin and ovarian development by chronic administration of testosterone in female Japanese silver eel, Anguilla japonica. Aquaculture 96(1):87–95. https://doi.org/10.1016/0044-8486(91)90141-S
Lokman PM, Wylie MJ, Downes M, Di Biase A, Damsteegt EL (2015) Artificial induction of maturation in female silver eels, Anguilla australis: the benefits of androgen pre-treatment. Aquaculture 437:111–119
Misawa A, Kada S, Yoshida M (2014) Comparison of the mode of action of three anesthetic agents, 2-phenoxyethanol, MS-222, and eugenol on goldfish. Aquac Sci 62(4):425–432
Monson C, Forsgren K, Goetz G, Harding L, Swanson P, Young G (2017) A teleost androgen promotes development of primary ovarian follicles in coho salmon and rapidly alters the ovarian transcriptome. Biol Reprod 97(5):731–745. https://doi.org/10.1093/biolre/iox124
Mordenti O, Emmanuele P, Casalini A, Lokman PM, Zaccaroni A, Di Biase A, Parmeggiani A (2018) Effect of aromatable androgen (17-methyltestosterone) on induced maturation of silver European eels (Anguilla Anguilla): oocyte performance and synchronization. Aquac Res 49:442–448
Okamura A, Horie N, Mikawa N, Yamada Y, Tsukamoto K (2014) Recent advances in artificial production of glass eels for conservation of anguillid eel populations. Ecol Freshw Fish 23:95–110
Rattan S, Flaws JA (2019) The epigenetic impacts of endocrine disruptors on female reproduction across generations. Biol Reprod 101(3):635–644. https://doi.org/10.1093/biolre/ioz081
Sudo R, Tosaka R, Ijiri S, Adachi S, Aoyama J, Tsukamoto K (2012) 11-ketotestosterone synchronously induces oocyte development and silvering-related changes in the Japanese eel, Anguilla japonica. Zool Sci 29(4):254–259
Tachiki H, Nakagawa T, Tamura K, Hirose K (1997) Effects of oral administration of estradiol-17 to young on gonadal sex and growth of Japanese eel Anguilla Japonica. Suisanzousyoku (水産増殖) 45(1):61–66 (in Japanese)
Tanaka H, Kagawa H, Ohta H, Unuma T, Nomura K (2003) The first production of glass eel in captivity: fish reproductive physiology facilitates great progress in aquaculture. Fish Physiol Biochem 28:493–497
Tanaka S, Okamura A, Mikawa N, Yamada Y, Horie N, Utoh T, Zhang H, Oka HP, Tsukamoto K (2018) Development of oocytes in a post-spawning Japanese eel Anguilla japonica Temminck & Schlegel in captivity. bioRxiv. https://doi.org/10.1101/494435
Thorstad EB, Økland F, Westerberg H, Aarestrup K, Metcalfe JD (2013) Evaluation of surgical implantation of electronic tags in European eel and effects of different suture materials. Mar Freshw Res 64:324–331
Tsukamoto K, Chow S, Otake T, Kurogi H, Mochioka N, Miller MJ, Aoyama J, Kimura S, Watanabe S, Yoshinaga T, Shinoda A, Kuroki M, Oya M, Watanabe T, Hata K, Ijiri S, Kazeto Y, Nomura K, Tanaka H (2011) Oceanic spawning ecology of freshwater eels in the western North Pacific. Nature Communications 2(1):179. https://doi.org/10.1038/ncomms1174
Tzeng WN, Han YS, He JT (2002) The sex ratios and growth strategies of wild and captive Japanese eels Anguilla japonica. In: Small B, MacKinlay D (eds) Developments in understanding fish growth, symposium proceedings of international congress of the biology of fish, University of British Columbia. Vancouver B.C, Canada, pp 25–42
Wang YS, Lou SW (2007) Influence of exogenous gonadotropin and sexual steroids on ovary development in Japanese eel, Anguilla japonica. J Fish Soc Taiwan 34:261–273. https://doi.org/10.29822/JFST.200709.0003
Walters KA, Middleton LJ, Joseph SR, Hazra R, Jimenez M, Simanainen U, Allan CM, Handelsman DJ (2012) Targeted loss of androgen receptor signaling in murine granulosa cells of preantral and antral follicles causes female subfertility. Biol Reprod 87(151):1–11
Wand MP, Jones MC (1994) Kernel smoothing. Chapman & Hall/CRC monographs on statistics & applied probability (60). Boca Raton, FL, U.S.: Chapman & Hall
Zaroogian GE, Gutjahr-Gobell RE, Horowitz DB, Jayaraman S, Cantwell M, Chichester CO, Mills LJ (2012) Injectable, slow-release implantation method for exposing fish to chemicals over a period of weeks. N Am J Aquac 74(4):512–521
Acknowledgments
We would like to thank Mr. Cheng Chang (Department of Life Science, NUK, Taiwan) for helping with the experimental manipulations, and Mr. YL Huang as well as Mr. YS Lei for their encouragement.
Funding
This study was partly funded by the Ministry of Science and Technology (MOST107-2321-B002-057), Taiwan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 704 kb)
Rights and permissions
About this article
Cite this article
Huang, YS., Wu, XH., Huang, PS. et al. Correlation between the ovarian status and the androgen sensibility in the cultured Japanese eel, Anguilla japonica. Fish Physiol Biochem 46, 1063–1074 (2020). https://doi.org/10.1007/s10695-020-00772-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-020-00772-1