Abstract
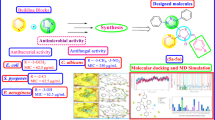
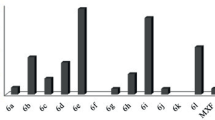
A library of pyrazole–thiazolidinone conjugates was synthesized using a molecular hybridization approach through a Vilsmeier–Haack reaction. The compounds were tested for anti-microbial activity against two Gram-positive bacteria (Staphylococcus aureus and methicillin-resistant Staphylococcus aureus) and four Gram-negative bacteria (Escherichia coli, Salmonella typhimurium, Klebsiella pneumonia and Pseudomonas aeruginosa). Among the compounds tested, 3-((2,4-dichlorophenyl)-1-(2,4-dinitrophenyl)-1H-pyrazol-yl)methylene)hydrazinecarbothioamide (3a) and 2-((3-(2-chlorophenyl)-1-(2,4 dinitrophenyl)-1H-pyrazol-4-yl)methyleneamino)thiazolidin-4-one (4b) emerged as the most potent anti-microbial compounds with minimum bactericidal concentrations of < 0.2 µM against MRSA and S. aureus. Structure–activity relationship analysis further revealed that the presence of 2,4-dichloro moiety surprisingly influenced the activity of the compounds. Molecular docking studies of the compounds into the crystal structure of topoisomerase II and topoisomerase IV suggest that compounds 3a and 4b preferably interact with the targets through hydrogen bonding.








Similar content being viewed by others
References
Tsogoeva SB (2010) Recent progress in the development of synthetic hybrids of natural or unnatural bioactive compounds for medicinal chemistry. Mini Rev Med Chem 10(9):773–793
Bhosle MR, Mali JR, Pal S, Srivastava AK, Mane RA (2014) Synthesis and antihyperglycemic evaluation of new 2-hydrazolyl-4-thiazolidinone-5-carboxylic acids having pyrazolyl pharmacophores. Bioorg Med Chem Lett 24(12):2651–2654
Helal M, Salem M, El-Gaby M, Aljahdali M (2013) Synthesis and biological evaluation of some novel thiazole compounds as potential anti-inflammatory agents. Eur J Med Chem 65:517–526
Tsuruoka A, Kaku Y, Kakinuma H, Tsukada I, Yanagisawa M, Nara K, Naito T (1998) Synthesis and antifungal activity of novel thiazole-containing triazole antifungals. II. Optically active ER-30346 and its derivatives. Chem Pharm Bull 46(4):623–630
Storer R, Ashton CJ, Baxter AD, Hann MM, Marr CL, Mason AM, Mo C-L, Myers PL, Noble SA, Penn CR (1999) The synthesis and antiviral activity of 4-fluoro-1-β-d-ribofuranosyl-1H-pyrazole-3-carboxamide. Nucleosides Nucleotides Nucleic Acids 18(2):203–216
Khunt R, Khedkar V, Chawda R, Chauhan N, Parikh A, Coutinho E (2012) Synthesis, antitubercular evaluation and 3D-QSAR study of N-phenyl-3-(4-fluorophenyl)-4-substituted pyrazole derivatives. Bioorg Med Chem Lett 22(1):666–678
Singh P, Mothilal S, Kerru N, Singh-Pillay A, Gummidi L, Erukainure OL, Islam MS (2019) Comparative α-glucosidase and α-amylase inhibition studies of rhodanine–pyrazole conjugates and their simple rhodanine analogues. Med Chem Res 28(2):143–159
Wu Y, Huang YP, Zhou SC, Tan YQ, Xu BG, Liang Z, Deng XQ (2018) Synthesis of 1, 3-diaryl pyrazole derivatives and evaluation of anticonvulsant and antimicrobial activities. Lat Am J Pharm 37(5):1017–1027
Sen S, De B, Easwari TS (2014) Synthesized 2-substituted-3-phenylthiazolidine-4-ones as potent antioxidants and antidiabetic agents. Trop J Pharm Res 13(9):1445–1454
Christiansen RG, Bell MR, D’Ambra TE, Mallamo JP, Herrmann JL, Ackerman JH, Opalka CJ, Kullnig RK, Winneker RC, Snyder BW (1990) Antiandrogenic steroidal sulfonylpyrazoles. J Med Chem 33(8):2094–2100
Thangamani S, Younis W, Seleem MN (2015) Repurposing celecoxib as a topical antimicrobial agent. Front Microbiol 6(6):750
Bekhit AA, Fahmy HT, Rostom SA, Baraka AM (2003) Design and synthesis of some substituted 1H-pyrazolyl-thiazolo [4, 5-d] pyrimidines as anti-inflammatory–antimicrobial agents. Eur J Med Chem 38(1):27–36
Ottana R, Maccari R, Barreca ML, Bruno G, Rotondo A, Rossi A, Chiricosta G, Di Paola R, Sautebin L, Cuzzocrea S (2005) 5-Arylidene-2-imino-4-thiazolidinones: design and synthesis of novel anti-inflammatory agents. Bioorg Med Chem 13(13):4243–4252
Omar K, Geronikaki A, Zoumpoulakis P, Camoutsis C, Soković M, Ćirić A, Glamočlija J (2010) Novel 4-thiazolidinone derivatives as potential antifungal and antibacterial drugs. Bioorg Med Chem 18(1):426–432
Mistry BM, Jauhari S (2013) Synthesis and in vitro antimicrobial and anti-tubercular evaluation of some quinoline-based azitidinone and thiazolidinone analogues. Med Chem Res 22(2):635–646
Hrib NJ, Jurcak JG, Bregna DE, Dunn RW, Geyer HM III, Hartman HB, Roehr JE, Rogers KL, Rush DK (1992) 3 [4-[1-(6-Fluorobenzo [b] thiophen-3-yl)-4-piperazinyl] butyl]-2, 5, 5-trimethyl-4-thiazolidinone: a new atypical antipsychotic agent for the treatment of schizophrenia. J Med Chem 35(14):2712–2715
Cai WX, Liu AL, Li ZM, Dong WL, Liu XH, Sun NB (2016) Synthesis and anticancer activity of novel thiazole-5-carboxamide derivatives. Appl Sci 6(1):8
Hambley TW (1997) The influence of structure on the activity and toxicity of Pt anti-cancer drugs. Coord Chem Rev 166:181–223
Gomha SM, Abdallah MA, Abbas IM, Kazem MS (2018) Synthesis, cytotoxicity evaluation, molecular docking and utility of novel chalcones as precursors for heterocycles incorporating pyrazole moiety. Med Chem 14(4):344–355
Ibrahim D (2009) Synthesis and biological evaluation of 3, 6-disubstituted [1, 2, 4] triazolo [3, 4-b][1, 3, 4] thiadiazole derivatives as a novel class of potential anti-tumor agents. Eur J Med Chem 44(7):2776–2781
Abdallah M, Gomha S, Abbas I, Kazem M, Alterary S, Mabkhot Y (2017) An efficient synthesis of novel pyrazole-based heterocycles as potential antitumor agents. Appl Sci 7(8):785
Abdel-Wahab BF, Mohamed SF, Amr AE-GE, Abdalla MM (2008) Synthesis and reactions of thiosemicarbazides, triazoles, and Schiff bases as antihypertensive α-blocking agents. Chem Mon 139(9):1083–1090
Bell SC, Wei P (1976) Syntheses of heterocylic fused thiazole acetic acids. 2. J Med Chem 19(4):524–530
Siddiqui N, Ahsan W (2011) Synthesis, anticonvulsant and toxicity screening of thiazolyl–thiadiazole derivatives. Med Chem Res 20(2):261–268
Bilbao-Ramos P, Galiana-Roselló C, Dea-Ayuela MA, González-Alvarez M, Vega C, Rolón M, Pérez-Serrano J, Bolás-Fernández F, González-Rosende ME (2012) Nuclease activity and ultrastructural effects of new sulfonamides with anti-leishmanial and trypanocidal activities. Parasitol Int 61(4):604–613
Badorc A, Bordes M-F, de Cointet P, Savi P, Bernat A, Lalé A, Petitou M, Maffrand J-P, Herbert J-M (1997) New orally active non-peptide fibrinogen receptor (GpIIb-IIIa) antagonists: identification of ethyl 3-[N-[4-[4-[amino [(ethoxycarbonyl) imino] methyl] phenyl]-1, 3-thiazol-2-yl]-N-[1-[(ethoxycarbonyl) methyl] piperid-4-yl] amino] propionate (SR 121787) as a potent and long-acting antithrombotic agent. J Med Chem 40(21):3393–3401
Küçükgüzel ŞG, Oruç EE, Rollas S, Şahin F, Özbek A (2002) Synthesis, characterisation and biological activity of novel 4-thiazolidinones, 1, 3, 4-oxadiazoles and some related compounds. Eur J Med Chem 37(3):197–206
Küçükgüzel G, Kocatepe A, De Clercq E, Şahin F, Güllüce M (2006) Synthesis and biological activity of 4-thiazolidinones, thiosemicarbazides derived from diflunisal hydrazide. Eur J Med Chem 41(3):353–359
Franklin TJ, Snow GA (2005) Biochemistry and molecular biology of antimicrobial drug action. Springer, Cham
Tocher JH (1997) Reductive activation of nitroheterocyclic compounds. Gener Pharmacol Vasc Syst 28(4):485–487
Liang B, Cheng H-Y, Kong D-Y, Gao S-H, Sun F, Cui D, Kong F-Y, Zhou A-J, Liu W-Z, Ren N-Q (2013) Accelerated reduction of chlorinated nitroaromatic antibiotic chloramphenicol by biocathode. Environ Sci Technol 47(10):5353–5361
Viegas-Junior C, Danuello A, da Silva Bolzani V, Barreiro EJ, Fraga CAM (2007) Molecular hybridization: a useful tool in the design of new drug prototypes. Curr Med Chem 14(17):1829–1852
Reece RJ, Maxwell A (1991) DNA gyrase: structure and function. Crit Rev Biochem Mol Biol 26(3–4):335–375
Collin F, Karkare S, Maxwell A (2011) Exploiting bacterial DNA gyrase as a drug target: current state and perspectives. Appl Microbiol Biotechnol 92(3):479–497
Maxwell A (1997) DNA gyrase as a drug target. Trends Microbiol 5(3):102–109
Yusufzai SK, Osman H, Khan MS, Razik BMA, Ezzat MO, Mohamad S, Sulaiman O, Gansau JA, Parumasivam TJCCJ (2018) 4-Thiazolidinone coumarin derivatives as two-component NS2B/NS3 DENV flavivirus serine protease inhibitors: synthesis, molecular docking, biological evaluation and structure–activity relationship studies. Chem Cent J 12(1):69
Dudek EP, Dudek GO (1967) Proton magnetic resonance spectra of thiocarboxamides. J Org Chem 32(3):823–824
Denis OR-V (2010) Hector; Struelens, Marc J: The problem of resistance. In: Finch Roger G (ed) Antibiotic and chemotherapy. Elsevier, Amsterdam, pp 24–48
Tanitame A, Oyamada Y, Ofuji K, Fujimoto M, Iwai N, Hiyama Y, Suzuki K, Ito H, Terauchi H, Kawasaki M (2004) Synthesis and antibacterial activity of a novel series of potent DNA gyrase inhibitors. Pyrazole derivatives. J Med Chem 47(14):3693–3696
Bennion BJ, Be NA, McNerney MW, Lao V, Carlson EM, Valdez CA, Malfatti MA, Enright HA, Nguyen TH, Lightstone FC (2017) Predicting a drug’s membrane permeability: a computational model validated with in vitro permeability assay data. J Phys Chem B 121(20):5228–5237
Alegaon SG, Hirpara M, Alagawadi K, Hullatti K, Kashniyal K (2014) Synthesis of novel pyrazole–thiadiazole hybrid as potential potent and selective cyclooxygenase-2 (COX-2) inhibitors. Bioorg Med Chem Lett 24(22):5324–5329
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25(13):1605–1612
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31(2):455–461
Salentin S, Schreiber S, Haupt VJ, Adasme MF, Schroeder M (2015) PLIP: fully automated protein–ligand interaction profiler. Nucleic Acids Res 43(W1):W443–W447
Acknowledgements
NK thanks the National Research Foundation South Africa for a Competitive Grant for Rated Researchers (Grant No. 118534) and Incentive Funding for Rated Researchers (Grant No. 114817). We would also like to thank the Centre for High Performance Computing based in Cape Town for access to computational resources.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ebenezer, O., Singh-Pillay, A., Koorbanally, N.A. et al. Antibacterial evaluation and molecular docking studies of pyrazole–thiosemicarbazones and their pyrazole–thiazolidinone conjugates. Mol Divers 25, 191–204 (2021). https://doi.org/10.1007/s11030-020-10046-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-020-10046-w