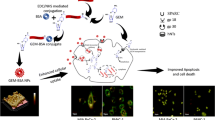
Abstract
Since the interactions of anti-cancer agents with blood constituents, in particular with human serum albumin (HSA) may have a major impact on drug pharmacology, the present study designed to provide a fundamental understanding of the interaction of nanodiamonds (NDs) together with paclitaxel (PTX) with HSA in detail for the first time. The UV–Vis, steady-state fluorescence, far-UV CD and zeta potential results displayed that PTX + NDs could form a complex with HSA. Additionally, the values of binding constants and ΔG° showed that PTX + NDs interact strongly with HSA compared to PTX or NDs alone and the hydrophobic force plays a major role in this interaction. Moreover, the in vitro release behavior of PTX + NDs form HSA can be regulated at dissimilar pH levels. The anticancer property of 0.5 µM PTX + 20 µM NDs by MTT assay demonstrates that this combination can tremendously diminish the proliferation of MDA-MB-231cells compared to PTX or NDs alone. Altogether, the structure of HSA changed moderately in the presence of PTX + NDs and PTX + NDs can promote mortality of MDA-MB-231 cells besides those mortality effects induced via PTX or NDs alone. The results obtained from this study can help in understanding the pharmacokinetic properties of PTX + NDs.









Similar content being viewed by others
References
El-Fakharany EM, Abu-Serie MM, Litus EA, Permyakov SE, Permyakov EA, Uversky VN, Redwan EM (2018) The use of human, bovine, and camel milk albumins in anticancer complexes with oleic acid. Protein J 37(3):203–215
Prado MA, Casado P, Zuazua-Villar P, del Valle E, Artime N, Cabal-Hierro L, Martínez-Campa CM, Lazo PS, Ramos S (2007) Phosphorylation of human eukaryotic elongation factor 1Bγ is regulated by paclitaxel. Proteomics 7(18):3299–3304
Garro A, Beltramo D, Alasino R, Leonhard V, Heredia V, Bianco I (2011) Reversible exposure of hydrophobic residues on albumin as a novel strategy for formulation of nanodelivery vehicles for taxanes. Int J Nanomed 6:1193–1200
Lee WL, Guo WM, Ho VH, Saha A, Chong HC, Tan NS, Tan EY, Loo SCJ (2015) Delivery of doxorubicin and paclitaxel from double-layered microparticles: the effects of layer thickness and dual-drug vs. single-drug loading. Acta Biomater 27:53–65
Hekmat A, Saboury AA, Divsalar A, Seyedarabi A (2013) Structural effects of TiO2 nanoparticles and doxorubicin on DNA and their antiproliferative roles in T47D and MCF7 cells. Anticancer Agents Med Chem 13(6):932–951
Vaijayanthimala V, Chang HC (2009) Functionalized fluorescent nanodiamonds for biomedical applications. Nanomedicine 4(1):47–55
Grall R, Girard H, Saad L, Petit T, Gesset C, Combis-Schlumberger M, Paget V, Delic J, Arnault JC, Chevillard S (2015) Impairing the radioresistance of cancer cells by hydrogenated nanodiamonds. Biomaterials 61:290–298
Brannon-Peppas L, Blanchette JO (2004) Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev 56(11):1649–1659
Keremidarska M, Ganeva A, Mitev D, Hikov T, Presker R, Pramatarova L, Krasteva N (2014) Comparative study of cytotoxicity of detonation nanodiamond particles with an osteosarcoma cell line and primary mesenchymal stem cells. Biotechnol Biotechnol Equip 28(4):733–739
Kaur R, Chitanda JM, Michel D, Maley J, Borondics F, Yang P, Verrall RE, Badea I (2012) Lysine-functionalized nanodiamonds: synthesis, physiochemical characterization, and nucleic acid binding studies. Int J Nanomedicine 7:3851–3866
Moosa B, Fhayli K, Li S, Julfakyan K, Ezzeddine A, Khashab NM (2014) Applications of nanodiamonds in drug delivery and catalysis. J Nanosci Nanotechnol 14(1):332–343
Chao J-I, Perevedentseva E, Chung P-H, Liu K-K, Cheng C-Y, Chang C-C, Cheng C-L (2007) Nanometer-sized diamond particle as a probe for biolabeling. Biophys J 93(6):2199–2208
Cheng C-Y, Perevedentseva E, Tu J-S, Chung P-H, Cheng C-L, Liu K-K, Chao J-I, Chen P-H, Chang C-C (2007) Direct and in vitro observation of growth hormone receptor molecules in A549 human lung epithelial cells by nanodiamond labeling. Appl Phys Lett 90(16):163903
Shimkunas RA, Robinson E, Lam R, Lu S, Xu X, Zhang X-Q, Huang H, Osawa E, Ho D (2009) Nanodiamond–insulin complexes as pH-dependent protein delivery vehicles. Biomaterials 30(29):5720–5728
Huang L-CL, Chang H-C (2004) Adsorption and immobilization of cytochrome c on nanodiamonds. Langmuir 20(14):5879–5884
Vial S, Mansuy C, Sagan S, Irinopoulou T, Burlina F, Boudou JP, Chassaing G, Lavielle S (2008) Peptide-grafted nanodiamonds: preparation, cytotoxicity and uptake in cells. ChemBioChem 9(13):2113–2119
Zhu Y, Zhang Y, Shi G, Yang J, Zhang J, Li W, Li A, Tai R, Fang H, Fan C (2015) Nanodiamonds act as Trojan horse for intracellular delivery of metal ions to trigger cytotoxicity. Part Fibre Toxicol 12(1):1–11
Purcell M, Neault JF, Tajmir-Riahi HA (2000) Interaction of taxol with human serum albumin. Biochim Biophys Acta 1478(1):61–68
Wang Y, Wang X, Wang J, Zhao Y, He W, Guo Z (2011) Noncovalent interactions between a trinuclear monofunctional platinum complex and human serum albumin. Inorg Chem 50(24):12661–12668
Liu K-K, Zheng W-W, Wang C-C, Chiu Y-C, Cheng C-L, Lo Y-S, Chen C, Chao J-I (2010) Covalent linkage of nanodiamond-paclitaxel for drug delivery and cancer therapy. Nanotechnology 21(31):315106
Chow EK, Zhang X-Q, Chen M, Lam R, Robinson E, Huang H, Schaffer D, Osawa E, Goga A, Ho D (2011) Nanodiamond therapeutic delivery agents mediate enhanced chemoresistant tumor treatment. Sci Transl Med 3(73):21
Xiao J, Duan X, Yin Q, Zhang Z, Yu H, Li Y (2013) Nanodiamonds-mediated doxorubicin nuclear delivery to inhibit lung metastasis of breast cancer. Biomaterials 34(37):9648–9656
Pashah Z, Hekmat A, Hesami Tackallou S (2019) Structural effects of diamond nanoparticles and paclitaxel combination on calf thymus DNA. Nucleosides Nucleotides Nucleic Acids 38(4):249–278
Maity S, Mukherjee K, Banerjee A, Mukherjee S, Dasgupta D, Gupta S (2016) Inhibition of porcine pancreatic amylase activity by sulfamethoxazole: structural and functional aspect. Protein J 35(3):237–246
Yuqin L, Guirong Y, Zhen Y, Caihong L, Baoxiu J, Jiao C, Yurong G (2014) Investigation of the interaction between patulin and human serum albumin by a spectroscopic method, atomic force microscopy, and molecular modeling. BioMed Res Int 2014:1–9
Na N, Zhao D-Q, Li H, Jiang N, Wen J-Y, Liu H-Y (2015) DNA binding, photonuclease activity and human serum albumin interaction of a water-soluble freebase carboxyl corrole. Molecules 21(1):1–14
He Y, Wang Y, Tang L, Liu H, Chen W, Zheng Z, Zou G (2008) Binding of puerarin to human serum albumin: a spectroscopic analysis and molecular docking. J Fluoresc 18(2):433–442
Li X, Wang G, Chen D, Lu Y (2014) Interaction of procyanidin B3 with bovine serum albumin. RSC Adv 4(14):7301–7312
Pan X, Qin P, Liu R, Wang J (2011) Characterizing the interaction between tartrazine and two serum albumins by a hybrid spectroscopic approach. J Agric Food Chem 59(12):6650–6656
Kalalbandi VKA, Seetharamappa J, Katrahalli U (2015) Synthesis, crystal studies and in vivo anti-hyperlipidemic activities of indole derivatives containing fluvastatin nucleus. RSC Adv 5(48):38748–38759
Yang X, Ye Z, Yuan Y, Zheng Z, Shi J, Ying Y, Huang P (2013) Insights into the binding of paclitaxel to human serum albumin: multispectroscopic studies. Luminescence 28(3):427–434
Karthikeyan S, Bharanidharan G, Kesherwani M, Mani KA, Srinivasan N, Velmurugan D, Aruna P, Ganesan S (2016) Insights into the binding of thiosemicarbazone derivatives with human serum albumin: Spectroscopy and molecular modelling studies. J Biomol Struct Dyn 34(6):1264–1281
Sahu SN, Padhan SK, Sahu PK (2016) Coumarin functionalized thiocarbonohydrazones as a new class of chromofluorescent receptors for selective detection of fluoride ion. RSC Adv 6(93):90322–90330
Veeralakshmi S, Sabapathi G, Nehru S, Venuvanalingam P, Arunachalam S (2017) Surfactant–cobalt (III) complexes: the impact of hydrophobicity on interaction with HSA and DNA–insights from experimental and theoretical approach. Colloids Surf B Biointerfaces 153:85–94
Hajihassan Z, Rabbani-Chadegani A (2011) The effect of mitoxantrone as an anticancer drug on hepatocytes nuclei and chromatin: selective release of histone proteins. Indian J Pharmacol 43(2):187–191
Amani N, Reza Saberi M, Khan Chamani J (2011) Investigation by fluorescence spectroscopy, resonance rayleigh scattering and zeta potential approaches of the separate and simultaneous binding effect of paclitaxel and estradiol with human serum albumin. Protein Pept Lett 18(9):935–951
Varlan A, Hillebrand M (2010) Bovine and human serum albumin interactions with 3-carboxyphenoxathiin studied by fluorescence and circular dichroism spectroscopy. Molecules 15(6):3905–3919
Zhou X, Lü W, Su L, Dong Y, Li Q, Chen X (2012) The binding affinity of amino acid–protein: hydroxyproline binding site I on human serum albumin. Org Biomol Chem 10(41):8314–8321
Šprung M, Soldo B, Orhanović S, Bučević-Popović V (2017) Substrate-induced conformational changes of the tyrocidine synthetase 1 adenylation domain probed by intrinsic Trp fluorescence. Protein J 36(3):202–211
Masters BR (2008) Principles of fluorescence spectroscopy. J Biomed Opt 13(2):029901
Moradi SZ, Nowroozi A, Sadrjavadi K, Moradi S, Mansouri K, Hosseinzadeh L, Shahlaei M (2018) Direct evidences for the groove binding of the Clomifene to double stranded DNA. Int J Biol Macromol 114:40–53
Yañez C, Günther G (2014) Is the determination of the association constant of cyclodextrin inclusion complexes dependent on the technique. J Chil Chem Soc 59(2):2523–2525
Shi J-H, Chen J, Wang J, Zhu Y-Y (2015) Binding interaction between sorafenib and calf thymus DNA: spectroscopic methodology, viscosity measurement and molecular docking. Spectrochim Acta A 136:443–450
Li S, Pan J, Zhang G, Xu J, Gong D (2017) Characterization of the groove binding between di-(2-ethylhexyl) phthalate and calf thymus DNA. Int J Biol Macromol 101:736–746
Hekmat A, Hajebrahimi Z, Motamedzade A (2017) Structural changes of human serum albumin (HSA) in simulated microgravity. Protein Pept Lett 24(11):1030–1039
Corrêa DH, Ramos CH (2009) The use of circular dichroism spectroscopy to study protein folding, form and function. Afr J Biochem Res 3(5):164–173
Wei Y, Thyparambil AA (1844) Latour RA (2014) Protein helical structure determination using CD spectroscopy for solutions with strong background absorbance from. Proteomics 12:2331–2337
Fu X-B, Liu D-D, Lin Y, Hu W, Mao Z-W, Le X-Y (2014) Water-soluble DNA minor groove binders as potential chemotherapeutic agents: synthesis, characterization, DNA binding and cleavage, antioxidation, cytotoxicity and HSA interactions. Dalton Trans 43(23):8721–8737
Gull N, Khan JM, Khan RH (2017) Spectroscopic studies on the gemini surfactant mediated refolding of human serum albumin. Int J Biol Macromol 102:331–335
Valstar A, Almgren M, Brown W, Vasilescu M (2000) The interaction of bovine serum albumin with surfactants studied by light scattering. Langmuir 16(3):922–927
Dasgupta N, Ranjan S, Patra D, Srivastava P, Kumar A, Ramalingam C (2016) Bovine serum albumin interacts with silver nanoparticles with a “side-on” or “end on” conformation. Chem Biol Interact 253:100–111
Li Y, Yang G, Mei Z (2012) Spectroscopic and dynamic light scattering studies of the interaction between pterodontic acid and bovine serum albumin. Acta Pharm Sin B 2(1):53–59
Ghosh D, Dey SK, Saha C (2014) Mutation induced conformational changes in genomic DNA from cancerous K562 cells influence drug-DNA binding modes. PLoS ONE 9(1):e84880
Sekowski S, Kazmierczak A, Mazur J, Przybyszewska M, Zaborski M, Shcharbin D, Gabryelak T (2009) The interaction between PAMAM G3 5 dendrimer, Cd2 +, dendrimer–Cd2 + complexes and human serum albumin. Colloids Surf B 69(1):95–98
Carter DC, Ho JX (1994) Structure of serum albumin. Adv Protein Chem 45:153–203
Hosainzadeh A, Gharanfoli M, Saberi MR, Chamani J (2012) Probing the interaction of human serum albumin with bilirubin in the presence of aspirin by multi-spectroscopic, molecular modeling and zeta potential techniques: insight on binary and ternary systems. J Biomol Struct Dyn 29(5):1013–1050
Omidvar Z, Parivar K, Sanee H, Amiri-Tehranizadeh Z, Baratian A, Saberi MR, Asoodeh A, Chamani J (2011) Investigations with spectroscopy, zeta potential and molecular modeling of the non-cooperative behaviour between cyclophosphamide hydrochloride and aspirin upon interaction with human serum albumin: Binary and ternary systems from the view point of multi-drug therapy. J Biomol Struct Dyn 29(1):181–206
Aramesh M, Shimoni O, Ostrikov K, Prawer S, Cervenka J (2015) Surface charge effects in protein adsorption on nanodiamonds. Nanoscale 7(13):5726–5736
Mochalin VN, Shenderova O, Ho D, Gogotsi Y (2012) The properties and applications of nanodiamonds. Nat Nanotechnol 7(1):11–23
Raghunand N, He X, Van Sluis R, Mahoney B, Baggett B, Taylor C, Paine-Murrieta G, Roe D, Bhujwalla ZM, Gillies R (1999) Enhancement of chemotherapy by manipulation of tumour pH. Br J Cancer 80(7):1005–1011
Gou Y, Zhang Z, Li D, Zhao L, Cai M, Sun Z, Li Y, Zhang Y, Khan H, Sun H (2018) HSA-based multi-target combination therapy: regulating drugs’ release from HSA and overcoming single drug resistance in a breast cancer model. Drug Deliv 25(1):321–329
Acknowledgements
We thank Ms. Evini at the Institute of Biochemistry and Biophysics of Tehran University for technical support. The authors thank Professor Gholamhossein Riazi (Institute of Biochemistry and Biophysics of Tehran University) for assistance with MTT assays.
Funding
This research received no specific funding and grant from any funding agency in the public, commercial, governmental, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hekmat, A., Salavati, F. & Hesami Tackallou, S. The Effects of Paclitaxel in the Combination of Diamond Nanoparticles on the Structure of Human Serum Albumin (HSA) and Their Antiproliferative Role on MDA-MB-231cells. Protein J 39, 268–283 (2020). https://doi.org/10.1007/s10930-020-09882-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-020-09882-4