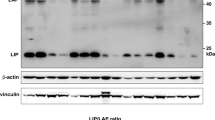
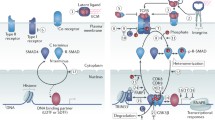
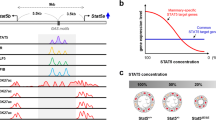
Abstract
It has been almost 30 years since C/EBPß was discovered. Seminal studies have shown that C/EBPß is a master regulator of mammary gland development and has been shown to control and influence proliferation and differentiation through varying mechanisms. The single-exon C/EBPß mRNA yields at least three different protein isoforms which have diverse, specific, context-dependent, and often non-overlapping roles throughout development and breast cancer progression. These roles are dictated by a number of complex factors including: expression levels of other C/EBP family members and their stoichiometry relative to the isoform in question, binding site affinity, post-translational modifications, co-factor expression, and even hormone levels and lactogenic status. Here we summarize the historical work up to the latest findings in the field on C/EBPß in the mammary gland and in breast cancer. With the current emphasis on improving immunotherapy in breast cancer the role of specific C/EBPß isoforms in regulating specific chemokine and cytokine expression and the immune microenvironment will be of increasing interest.




Similar content being viewed by others
References
Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J. 2002;365:561–75 Available from: http://www.ncbi.nlm.nih.gov/pubmed/12006103.
Ma D, Panda S, Lin JD. Temporal orchestration of circadian autophagy rhythm by C/EBPβ. EMBO J. 2011;30:4642–51 Available from: http://emboj.embopress.org/cgi/doi/10.1038/emboj.2011.322.
Pham T, Langmann S, Schwarzfischer L, El Chartouni C, Lichtinger M, Klug M, et al. CCAAT enhancer-binding protein beta regulates constitutive gene expression during late stages of monocyte to macrophage differentiation. J Biol Chem. 2007;282:21924–33 Available from: http://www.ncbi.nlm.nih.gov/pubmed/17540774.
Carro MS, Lim WK, Alvarez MJ, Bollo RJ, Zhao X, Snyder EY, et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature. Nature Publishing Group; 2010;463:318–325. https://doi.org/10.1038/nature08712
Luedi MM, Singh SK, Mosley JC, Hatami M, Gumin J, Sulman EP, et al. A Dexamethasone-regulated Gene Signature Is Prognostic for Poor Survival in Glioblastoma Patients. J Neurosurg Anesthesiol. 2017;29:46–58 Available from: http://www.ncbi.nlm.nih.gov/pubmed/27653222.
Wilson S, Filipp FV. A network of epigenomic and transcriptional cooperation encompassing an epigenomic master regulator in cancer. NPJ Syst Biol Appl. Springer US. 2018;4:24. https://doi.org/10.1038/s41540-018-0061-4.
Zaret KS, Carroll JS. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 2011;25:2227–41 Available from: http://www.ncbi.nlm.nih.gov/pubmed/22056668.
Iyer VV, Kadakia TB, McCabe LR, Schwartz RC. CCAAT/enhancer-binding protein-β has a role in osteoblast proliferation and differentiation. Exp Cell Res. 2004;295:128–37.
Müller C, Kowenz-Leutz E, Grieser-Ade S, Graf T, Leutz A. NF-M (chicken C/EBP beta) induces eosinophilic differentiation and apoptosis in a hematopoietic progenitor cell line. EMBO J. 1995;14:6127–35 Available from: http://www.ncbi.nlm.nih.gov/pubmed/8557032.
Pan Z, Hetherington CJ, Zhang DE. CCAAT/enhancer-binding protein activates the CD14 promoter and mediates transforming growth factor beta signaling in monocyte development. J Biol Chem. 1999;274:23242–8 Available from: http://www.ncbi.nlm.nih.gov/pubmed/10438498.
Lamkin DM, Srivastava S, Bradshaw KP, Betz JE, Muy KB, Wiese AM, et al. C/EBPβ regulates the M2 transcriptome in β-adrenergic-stimulated macrophages. Brain Behav Immun. Elsevier. 2019;80:839–48. https://doi.org/10.1016/j.bbi.2019.05.034.
Yoshioka S, Miura Y, Yao H, Satake S, Hayashi Y, Tamura A, et al. CCAAT/enhancer-binding protein β expressed by bone marrow mesenchymal stromal cells regulates early B-cell lymphopoiesis. Stem Cells. 2014;32:730–40 Available from: http://www.ncbi.nlm.nih.gov/pubmed/24115241.
Kowenz-Leutz E, Leutz A. A C/EBPβ Isoform Recruits the SWI/SNF Complex to Activate Myeloid Genes. Mol Cell. 1999;4:735–43 Available from: http://www.ncbi.nlm.nih.gov/pubmed/10619021.
Smink JJ, Bégay V, Schoenmaker T, Sterneck E, De Vries TJ, Leutz A. Transcription factor C/EBPΒ isoform ratio regulates osteoclastogenesis through MafB. EMBO J. 2009;28:1769–81.
Darlington GJ. Molecular mechanisms of liver development and differentiation. Curr Opin Cell Biol. 1999;11:678–82 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0955067499000356.
Welm AL, Timchenko NA, Darlington GJ. C/EBPalpha regulates generation of C/EBPbeta isoforms through activation of specific proteolytic cleavage. Mol Cell Biol. 1999;19:1695–704 Available from: http://www.ncbi.nlm.nih.gov/pubmed/10022857.
Ferrini JB, Rodrigues E, Dulic V, Pichard-Garcia L, Fabr JM, Blanc P, et al. Expression and DNA-binding activity of C/EBPalpha and C/EBPbeta in human liver and differentiated primary hepatocytes. J Hepatol. 2001;35:170–7 Available from: http://www.ncbi.nlm.nih.gov/pubmed/11580138.
Maytin EV, Habener JF. Transcription factors C/EBP alpha, C/EBP beta, and CHOP (Gadd153) expressed during the differentiation program of keratinocytes in vitro and in vivo. J invest Dermatol. Elsevier Masson SAS. 1998;110:238–46. https://doi.org/10.1046/j.1523-1747.1998.00123.x.
Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991;5:1538–52 Available from: http://www.ncbi.nlm.nih.gov/pubmed/1840554.
Manchado C, Yubero P, Viñas O, Iglesias R, Villarroya F, Mampel T, et al. CCAAT/enhancer-binding proteins alpha and beta in brown adipose tissue: evidence for a tissue-specific pattern of expression during development. Biochem J. 1994;302 ( Pt 3:695–700. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7945193.
Sterneck E, Tessarollo L, Johnson PF. An essential role for C/EBPbeta in female reproduction. Genes Dev. 1997;11:2153–62 Available from: http://www.ncbi.nlm.nih.gov/pubmed/9303532.
Fan H-Y, Liu Z, Johnson PF, Richards JS. CCAAT/enhancer-binding proteins (C/EBP)-α and -β are essential for ovulation, luteinization, and the expression of key target genes. Mol Endocrinol. 2011;25:253–68 Available from: http://www.ncbi.nlm.nih.gov/pubmed/21177758.
Wang W, Taylor RN, Bagchi IC, Bagchi MK. Regulation of human endometrial stromal proliferation and differentiation by C/EBPβ involves cyclin E-cdk2 and STAT3. Mol Endocrinol. 2012 [cited 2013 Jun 26];26:2016–30. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23097472.
Robinson GW, Johnson PF, Hennighausen L, Sterneck E. The C/EBPbeta transcription factor regulates epithelial cell proliferation and differentiation in the mammary gland. Genes Dev. 1998;12:1907–16 Available from: http://www.ncbi.nlm.nih.gov/pubmed/9637691.
Seagroves TN, Krnacik S, Raught B, Gay J, Burgess-Beusse B, Darlington GJ, et al. C/EBPbeta , but not C/EBPalpha , is essential for ductal morphogenesis, lobuloalveolar proliferation, and functional differentiation in the mouse mammary gland. Genes Dev. 1998;12:1917–28 Available from: http://www.ncbi.nlm.nih.gov/pubmed/9637692.
Seagroves TN, Lydon JP, Hovey RC, Vonderhaar BK, Rosen JM. C/EBPβ (CCAAT/Enhancer Binding Protein) Controls Cell Fate Determination during Mammary Gland Development. Mol Endocrinol. 2000;14:359–68 Available from: http://www.ncbi.nlm.nih.gov/pubmed/10707954.
LaMarca HL, Visbal AP, Creighton CJ, Liu H, Zhang Y, Behbod F, et al. CCAAT/enhancer binding protein beta regulates stem cell activity and specifies luminal cell fate in the mammary gland. Stem Cells. 2010 [cited 2013 Jun 26];28:535–44. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3006225&tool=pmcentrez&rendertype=abstract
Doppler W, Welte T, Philipp S. CCAAT/enhancer-binding protein isoforms beta and delta are expressed in mammary epithelial cells and bind to multiple sites in the beta-casein gene promoter. J Biol Chem. 1995;270:17962–9 Available from: http://www.ncbi.nlm.nih.gov/pubmed/7629103.
Isshiki H, Akira S, Tanabe O, Nakajima T, Shimamoto T, Hirano T, et al. Constitutive and interleukin-1 (IL-1)-inducible factors interact with the IL-1-responsive element in the IL-6 gene. Mol Cell Biol. 1990;10:2757–64 Available from: http://www.ncbi.nlm.nih.gov/pubmed/2111442.
Akira S, Isshiki H, Sugita T, Tanabe O, Kinoshita S, Nishio Y, et al. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. Embo J. 1990;9:1897–906 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=551896&tool=pmcentrez&rendertype=abstract.
Descombes P, Chojkier M, Lichtsteiner S, Falvey E, Schibler U. LAP, a novel member of the C/EBP gene family, encodes a liver-enriched transcriptional activator protein. Genes Dev. 1990;4:1541–51 Available from: http://www.ncbi.nlm.nih.gov/pubmed/2253878.
Descombes P, Schibler U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell. 1991;67:569–79 Available from: http://www.ncbi.nlm.nih.gov/pubmed/1934061.
Calkhoven CF, Müller C, Leutz A. Translational control of C/EBPalpha and C/EBPbeta isoform expression. Genes Dev. 2000;14:1920–32 Available from: http://www.ncbi.nlm.nih.gov/pubmed/10921906.
LeClair KP, Blanar MA, Sharp PA. The p50 subunit of NF-kappa B associates with the NF-IL6 transcription factor. Proc Natl Acad Sci U S A. 1992;89:8145–9 Available from: http://www.ncbi.nlm.nih.gov/pubmed/1518839.
Matsusaka T, Fujikawa K, Nishio Y, Mukaida N, Matsushima K, Kishimoto T, et al. Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci U S A. 1993;90:10193–7 Available from: http://www.ncbi.nlm.nih.gov/pubmed/8234276.
Nishio Y, Isshiki H, Kishimoto T, Akira S. A nuclear factor for interleukin-6 expression (NF-IL6) and the glucocorticoid receptor synergistically activate transcription of the rat alpha 1-acid glycoprotein gene via direct protein-protein interaction. Mol Cell Biol. 1993;13:1854–62 Available from: http://www.ncbi.nlm.nih.gov/pubmed/8441418.
Hsu W, Kerppola TK, Chen PL, Curran T, Chen-Kiang S. Fos and Jun repress transcription activation by NF-IL6 through association at the basic zipper region. Mol Cell Biol. 1994;14:268–76 Available from: http://www.ncbi.nlm.nih.gov/pubmed/8264594.
Zhang Y, Rom WN. Regulation of the interleukin-1 beta (IL-1 beta) gene by mycobacterial components and lipopolysaccharide is mediated by two nuclear factor-IL6 motifs. Mol Cell Biol. 1993;13:3831–7 Available from: http://www.ncbi.nlm.nih.gov/pubmed/7684503.
Stein B, Baldwin AS. Distinct mechanisms for regulation of the interleukin-8 gene involve synergism and cooperativity between C/EBP and NF-kappa B. Mol Cell Biol. 1993;13:7191–8 Available from: http://www.ncbi.nlm.nih.gov/pubmed/8413306.
Dunn SM, Coles LS, Lang RK, Gerondakis S, Vadas MA, Shannon MF. Requirement for nuclear factor (NF)-kappa B p65 and NF-interleukin-6 binding elements in the tumor necrosis factor response region of the granulocyte colony-stimulating factor promoter. Blood. 1994;83:2469–79 Available from: http://www.ncbi.nlm.nih.gov/pubmed/7513199.
Mukaida N, Morita M, Ishikawa Y, Rice N, Okamoto S, Kasahara T, et al. Novel mechanism of glucocorticoid-mediated gene repression. Nuclear factor-kappa B is target for glucocorticoid-mediated interleukin 8 gene repression. J Biol Chem. 1994;269:13289–95 Available from: http://www.ncbi.nlm.nih.gov/pubmed/8175759.
Davydov IV, Krammer PH, Li-Weber M. Nuclear factor-IL6 activates the human IL-4 promoter in T cells. J Immunol. 1995;155:5273–9 Available from: http://www.ncbi.nlm.nih.gov/pubmed/7594540.
Wethmar K, Bégay V, Smink JJ, Zaragoza K, Wiesenthal V, Dörken B, et al. C/EBPβΔuORF mice - a genetic model for uORF-mediated translational control in mammals. Genes Dev. 2010;24:15–20.
Bégay V, Smink JJ, Loddenkemper C, Zimmermann K, Rudolph C, Scheller M, et al. Deregulation of the endogenous C/EBPβ LIP isoform predisposes to tumorigenesis. J Mol Med. 2015;93:39–49.
Zidek LM, Ackermann T, Hartleben G, Eichwald S, Kortman G, Kiehntopf M, et al. Deficiency in mTORC 1-controlled C/ EBP β - mRNA translation improves metabolic health in mice. EMBO Rep. 2015;16:1022–36.
Müller C, Zidek LM, Ackermann T, de Jong T, Liu P, Kliche V, et al. Reduced expression of C/EBPβ-LIP extends health and lifespan in mice. Elife. 2018;7:1–28.
Dittmar G, Hernandez DP, Kowenz-Leutz E, Kirchner M, Kahlert G, Wesolowski R, et al. PRISMA: Protein Interaction Screen on Peptide Matrix Reveals Interaction Footprints and Modifications- Dependent Interactome of Intrinsically Disordered C/EBPβ. iScience. 2019;13:351–70 Available from: https://linkinghub.elsevier.com/retrieve/pii/S2589004219300604.
Tang Q, Grønborg M, Huang H, Kim J, Otto TC, Pandey A, et al. Sequential phosphorylation of CCAAT enhancer-binding protein beta by MAPK and glycogen synthase kinase 3beta is required for adipogenesis. Proc Natl Acad Sci U S A. 2005;102:9766–71 Available from: http://www.ncbi.nlm.nih.gov/pubmed/15985551.
Kim J, Tang Q, Li X, Lane MD. Effect of phosphorylation and S-S bond-induced dimerization on DNA binding and transcriptional activation by C/EBPbeta. Proc Natl Acad Sci U S A. 2007;104:1800–4 Available from: http://www.ncbi.nlm.nih.gov/pubmed/17264204.
Hattori T, Ohoka N, Inoue Y, Hayashi H, Onozaki K. C/EBP family transcription factors are degraded by the proteasome but stabilized by forming dimer. Oncogene. 2003;22:1273–80 Available from: http://www.ncbi.nlm.nih.gov/pubmed/12618752.
Zhang Y, Li S, Qian S, Zhang Y, Liu Y, Tang Q-Q, et al. Phosphorylation prevents C/EBPβ from the calpain-dependent degradation. Biochem Biophys Res Commun. 2012;419:550–5 Available from: http://www.ncbi.nlm.nih.gov/pubmed/22369944.
Li X, Molina H, Huang H, Zhang Y, Liu M, Qian S, et al. O-linked N-acetylglucosamine modification on CCAAT enhancer-binding protein beta: role during adipocyte differentiation. J Biol Chem. 2009;284:19248–54 Available from: http://www.ncbi.nlm.nih.gov/pubmed/19478079.
Ceseña TI, Cardinaux J-R, Kwok R, Schwartz J. CCAAT/enhancer-binding protein (C/EBP) beta is acetylated at multiple lysines: acetylation of C/EBPbeta at lysine 39 modulates its ability to activate transcription. J Biol Chem. 2007 [cited 2013 Jun 26];282:956–67. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17110376.
Ceseña TI, Cui TX, Subramanian L, Fulton CT, Iñiguez-Lluhí JA, Kwok RPS, et al. Acetylation and deacetylation regulate CCAAT/enhancer binding protein β at K39 in mediating gene transcription. Mol Cell Endocrinol. 2008;289:94–101.
Wiper-Bergeron N, Salem HA, Tomlinson JJ, Wu D, Haché RJG. Glucocorticoid-stimulated preadipocyte differentiation is mediated through acetylation of C/EBPbeta by GCN5. Proc Natl Acad Sci U S A. 2007;104:2703–8 Available from: http://www.ncbi.nlm.nih.gov/pubmed/17301242.
Xu M, Nie L, Kim S, Sun X. STAT5-induced Id-1 transcription involves recruitment of HDAC1 and deacetylation of C/EBPbeta. EMBO J. 2003;22:893–904 Available from: http://www.ncbi.nlm.nih.gov/pubmed/12574125.
Pless O, Kowenz-Leutz E, Knoblich M, Lausen J, Beyermann M, Walsh MJ, et al. G9a-mediated lysine methylation alters the function of CCAAT/enhancer-binding protein-beta. J Biol Chem. 2008;283:26357–63 Available from: http://www.ncbi.nlm.nih.gov/pubmed/1864779.
Kowenz-Leutz E, Pless O, Dittmar G, Knoblich M, Leutz A. Crosstalk between C/EBPbeta phosphorylation, arginine methylation, and SWI/SNF/Mediator implies an indexing transcription factor code. EMBO J . Nature Publishing Group; 2010 [cited 2013 Jun 18];29:1105–15. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2845275&tool=pmcentrez&rendertype=abstract
Kim J, Cantwell CA, Johnson PF, Pfarr CM, Williams SC. Transcriptional Activity of CCAAT / Enhancer-binding Proteins Is Controlled by a Conserved Inhibitory Domain That Is a Target for Sumoylation *. 2002;277:38037–44.
Eaton EM, Sealy L. Modification of CCAAT/enhancer-binding protein-beta by the small ubiquitin-like modifier (SUMO) family members, SUMO-2 and SUMO-3. J Biol Chem. 2003;278:33416–21 Available from: http://www.ncbi.nlm.nih.gov/pubmed/12810706.
Subramanian L, Benson MD, In JA. A Synergy Control Motif within the Attenuator Domain of CCAAT / Enhancer-binding Protein ␣ Inhibits Transcriptional Synergy through Its PIASy-enhanced Modification by SUMO-1 or SUMO-3 *. 2003;278:9134–41.
Berberich-Siebelt F, Berberich I, Andrulis M, Santner-Nanan B, Jha MK, Klein-Hessling S, et al. SUMOylation interferes with CCAAT/enhancer-binding protein beta-mediated c-myc repression, but not IL-4 activation in T cells. J Immunol. 2006;176:4843–51 Available from: http://www.ncbi.nlm.nih.gov/pubmed/16585579.
Liu Y, Zhang Y, Guo L, Huang H-Y, Zhu H, Huang J, et al. Protein inhibitor of activated STAT 1 (PIAS1) is identified as the SUMO E3 ligase of CCAAT/enhancer-binding protein β (C/EBPβ) during adipogenesis. Mol Cell Biol. 2013;33:4606–17 Available from: http://www.ncbi.nlm.nih.gov/pubmed/24061474.
Cong L, Zhang F. Genome engineering using CRISPR-Cas9 system. Methods Mol Biol. 2015;1239:197–217 Available from: http://www.ncbi.nlm.nih.gov/pubmed/25408407.
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–23 Available from: http://www.ncbi.nlm.nih.gov/pubmed/23287718.
Kabotyanski EB, Huetter M, Xian W, Rijnkels M, Rosen JM. Integration of prolactin and glucocorticoid signaling at the beta-casein promoter and enhancer by ordered recruitment of specific transcription factors and chromatin modifiers. Mol Endocrinol. 2006;20:2355–68 Available from: http://www.ncbi.nlm.nih.gov/pubmed/16772529.
Christian M, Pohnke Y, Kempf R, Gellersen B, Brosens JJ. Functional association of PR and CCAAT/enhancer-binding protein beta isoforms: promoter-dependent cooperation between PR-B and liver-enriched inhibitory protein, or liver-enriched activatory protein and PR-A in human endometrial stromal cells. Mol Endocrinol. 2002;16:141–54 Available from: http://www.ncbi.nlm.nih.gov/pubmed/11773445.
Liu Q, Boudot A, Ni J, Hennessey T, Beauparlant SL, Rajabi HN, et al. Cyclin D1 and C/EBPβ LAP1 operate in a common pathway to promote mammary epithelial cell differentiation. Mol Cell Biol. 2014;34:3168–79 Available from: http://mcb.asm.org/lookup/doi/10.1128/MCB.00039-14.
Spooner CJ, Guo X, Johnson PF, Schwartz RC. Differential roles of C/EBP beta regulatory domains in specifying MCP-1 and IL-6 transcription. Mol Immunol. 2007;44:1384–92 Available from: http://www.ncbi.nlm.nih.gov/pubmed/16784777.
Gomis RR, Alarcón C, Nadal C, Van Poznak C, Massagué J. C/EBPβ at the core of the TGFβ cytostatic response and its evasion in metastatic breast cancer cells. Cancer Cell . 2006 [cited 2013 Jun 6];10:203–14. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16959612.
Wang H, Larris B, Peiris TH, Zhang L, Le Lay J, Gao Y, et al. C/EBPbeta activates E2F-regulated genes in vivo via recruitment of the coactivator CREB-binding protein/P300. J Biol Chem . 2007 [cited 2013 Jun 26];282:24679–88. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17599912.
Davis CA, Hitz BC, Sloan CA, Chan ET, Davidson JM, Gabdank I, et al. The Encyclopedia of DNA elements (ENCODE): data portal update. Nucleic Acids Res . Oxford University Press; 2018;46:D794–801. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29126249.
Raught B, Liao WS-L, Rosen JM. Developmentally and hormonally regulated CCAAT/enhancer-binding protein isoforms influence beta-casein gene expression. Mol Endocrinol. 1995;9:1223–32 Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7491114.
Wang W, Do HN, Aupperlee MD, Durairaj S, Flynn EE, Miksicek RJ, et al. C/EBPβ LIP and c-Jun synergize to regulate expression of the murine progesterone receptor. Mol Cell Endocrinol . Elsevier. 2018;477:57–69. https://doi.org/10.1016/j.mce.2018.06.001.
Kabotyanski EB, Rijnkels M, Freeman-Zadrowski C, Buser AC, Edwards DP, Rosen JM. Lactogenic hormonal induction of long distance interactions between beta-casein gene regulatory elements. J Biol Chem. 2009;284:22815–24 Available from: http://www.ncbi.nlm.nih.gov/pubmed/19542223.
Park B, Kook S, Lee S, Jeong J, Brufsky A, Lee B. An isoform of C/EBPβ, LIP, regulates expression of the chemokine receptor CXCR4 and modulates breast cancer cell migration. J Biol Chem. 2013;288:28656–67 Available from: http://www.ncbi.nlm.nih.gov/pubmed/23966000.
Abraham S, Sweet T, Sawaya BE, Rappaport J, Khalili K, Amini S. Cooperative interaction of C/EBP beta and Tat modulates MCP-1 gene transcription in astrocytes. J Neuroimmunol. 2005;160:219–27 Available from: http://www.ncbi.nlm.nih.gov/pubmed/15710476.
Raught B, Gingras AC, James A, Medina D, Sonenberg N, Rosen JM. Expression of a translationally regulated, dominant-negative CCAAT/enhancer-binding protein beta isoform and up-regulation of the eukaryotic translation initiation factor 2alpha are correlated with neoplastic transformation of mammary epithelial cells. Cancer Res. 1996;56:4382–6 Available from: http://www.ncbi.nlm.nih.gov/pubmed/8813130.
Gigliotti AP, DeWille JW. Lactation status influences expression of CCAAT/enhancer binding protein isoform mRNA in the mouse mammary gland. J Cell Physiol. 1998;174:232–9 Available from: http://www.ncbi.nlm.nih.gov/pubmed/9428809.
Eaton EM, Hanlon M, Bundy L, Sealy L. Characterization of C/EBPbeta isoforms in normal versus neoplastic mammary epithelial cells. J Cell Physiol. 2001;189:91–105 Available from: http://www.ncbi.nlm.nih.gov/pubmed/11573208.
Dearth LR, Hutt J, Sattler A, Gigliotti A, DeWille J. Expression and function of CCAAT/enhancer binding protein? (C/EBP?) LAP and LIP isoforms in mouse mammary gland, tumors and cultured mammary epithelial cells. J Cell Biochem. 2001;82:357–70 Available from: http://www.ncbi.nlm.nih.gov/pubmed/11500913.
Zahnow CA, Younes P, Laucirica R, Rosen JM. Overexpression of C/EBPbeta-LIP, a naturally occurring, dominant-negative transcription factor, in human breast cancer. J Natl Cancer Inst. 1997;89:1887–91 Available from: https://academic.oup.com/jnci/article-lookup/doi/10.1093/jnci/89.24.1887.
Zahnow CA, Cardiff RD, Laucirica R, Medina D, Rosen JM. A role for CCAAT/enhancer binding protein beta-liver-enriched inhibitory protein in mammary epithelial cell proliferation. Cancer Res. 2001;61:261–9 Available from: http://www.ncbi.nlm.nih.gov/pubmed/11196172.
Bundy LM, Sealy L. CCAAT/enhancer binding protein beta (C/EBPbeta)-2 transforms normal mammary epithelial cells and induces epithelial to mesenchymal transition in culture. Oncogene. 2003;22:869–83 Available from: http://www.ncbi.nlm.nih.gov/pubmed/12584567.
Bundy L, Wells S, Sealy L. C/EBPbeta-2 confers EGF-independent growth and disrupts the normal acinar architecture of human mammary epithelial cells. Mol Cancer. 2005;4:43 Available from: http://www.ncbi.nlm.nih.gov/pubmed/16371159.
Miura Y, Hagiwara N, Radisky DC, Hirai Y. CCAAT/enhancer binding protein beta (C/EBPβ) isoform balance as a regulator of epithelial-mesenchymal transition in mouse mammary epithelial cells. Exp Cell Res . Elsevier. 2014;327:146–55. https://doi.org/10.1016/j.yexcr.2014.05.019.
Russell A, Boone B, Jiang A, Sealy L. Genomic profiling of C/EBPβ2 transformed mammary epithelial cells: a role for nuclear interleukin-1β. Cancer Biol Ther. 2010;10:509–19 Available from: http://www.ncbi.nlm.nih.gov/pubmed/21057224.
Jundt F, Raetzel N, Müller C, Calkhoven CF, Kley K, Mathas S, et al. A rapamycin derivative (everolimus) controls proliferation through down-regulation of truncated CCAAT enhancer binding protein {beta} and NF-{kappa}B activity in Hodgkin and anaplastic large cell lymphomas. Blood . 2005 [cited 2014 mar 17];106:1801–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15886325.
Benz CC, Scott GK, Santos GF, Smith HS. Expression of c-myc, c-Ha-ras1, and c-erbB-2 proto-oncogenes in normal and malignant human breast epithelial cells. J Natl Cancer Inst. 1989;81:1704–9 Available from: http://www.ncbi.nlm.nih.gov/pubmed/2572702.
Timchenko NA, Welm AL, Lu X, Timchenko LT. CUG repeat binding protein (CUGBP1) interacts with the 5. 1999;27:1–9. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC148737/pdf/274517.pdf%5Cnpapers3://publication/uuid/4D31EAFB-579C-444A-9E80-43A9663E4B5A
Baldwin BR, Timchenko NA, Zahnow CA. Epidermal growth factor receptor stimulation activates the RNA binding protein CUG-BP1 and increases expression of C/EBPbeta-LIP in mammary epithelial cells. Mol Cell Biol. 2004;24:3682–91 Available from: http://www.ncbi.nlm.nih.gov/pubmed/15082764.
Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res. 1998;58:2825–31 Available from: http://www.ncbi.nlm.nih.gov/pubmed/9661897.
Arnal-Estapé A, Tarragona M, Morales M, Guiu M, Nadal C, Massagué J, et al. HER2 silences tumor suppression in breast cancer cells by switching expression of C/EBPß isoforms. Cancer Res . 2010 [cited 2013 Jun 6];70:9927–36. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21098707.
Gustafson TL, Wellberg E, Laffin B, Schilling L, Metz RP, Zahnow CA, et al. Ha-Ras transformation of MCF10A cells leads to repression of Singleminded-2s through NOTCH and C/EBPbeta. Oncogene. 2009;28:1561–8 Available from: http://www.ncbi.nlm.nih.gov/pubmed/19169276.
Johansson J, Berg T, Kurzejamska E, Pang M, Tabor V, Jansson M, et al. MiR-155-mediated loss of C/EBPβ shifts the TGF-β response from growth inhibition to epithelial-mesenchymal transition, invasion and metastasis in breast cancer. Oncogene . Nature Publishing Group. 2013;32:5614–24. https://doi.org/10.1038/onc.2013.322.
Kurzejamska E, Johansson J, Jirström K, Prakash V, Ananthaseshan S, Boon L, et al. C/EBPβ expression is an independent predictor of overall survival in breast cancer patients by MHCII/CD4-dependent mechanism of metastasis formation. Oncogenesis. 2014;3:e125 http://www.nature.com/articles/oncsis201438.
Willis S, De P, Dey N, Long B, Young B, Sparano JA, et al. Enriched transcription factor signatures in triple negative breast cancer indicates possible targeted therapies with existing drugs. Meta gene . Elsevier B.V. 2015;4:129–41. https://doi.org/10.1016/j.mgene.2015.04.002.
Jinesh GG, Flores ER, Brohl AS. Chromosome 19 miRNA cluster and CEBPB expression specifically mark and potentially drive triple negative breast cancers. Ahmad A, editor. PLoS One . 2018;13:e0206008. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30335837.
Li W, Tanikawa T, Kryczek I, Xia H, Li G, Wu K, et al. Aerobic Glycolysis Controls Myeloid-Derived Suppressor Cells and Tumor Immunity via a Specific CEBPB Isoform in Triple-Negative Breast Cancer. Cell Metab. 2018;28:87–103.e6 Available from: http://www.ncbi.nlm.nih.gov/pubmed/29805099.
Salaroglio IC, Gazzano E, Abdullrahman A, Mungo E, Castella B, Abd-Elrahman GEFA-E, et al. Increasing intratumor C/EBP-β LIP and nitric oxide levels overcome resistance to doxorubicin in triple negative breast cancer. J Exp Clin Cancer Res . Journal of Experimental & Clinical Cancer Research; 2018;37:286. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30482226.
Tsukada J, Saito K, Waterman WR, Webb AC, Auron PE. Transcription factors NF-IL6 and CREB recognize a common essential site in the human prointerleukin 1 beta gene. Mol Cell Biol. 1994;14:7285–97 Available from: http://www.ncbi.nlm.nih.gov/pubmed/7935442.
Grove M, Plumb M. C/EBP, NF-kappa B, and c-Ets family members and transcriptional regulation of the cell-specific and inducible macrophage inflammatory protein 1 alpha immediate-early gene. Mol Cell Biol. 1993;13:5276–89 Available from: http://www.ncbi.nlm.nih.gov/pubmed/8355682.
Pope RM, Leutz A, Ness SA. C/EBP beta regulation of the tumor necrosis factor alpha gene. J Clin Invest. 1994;94:1449–55 Available from: http://www.ncbi.nlm.nih.gov/pubmed/7929820.
Nomiyama H, Hieshima K, Hirokawa K, Hattori T, Takatsuki K, Miura R. Characterization of cytokine LD78 gene promoters: positive and negative transcriptional factors bind to a negative regulatory element common to LD78, interleukin-3, and granulocyte-macrophage colony-stimulating factor gene promoters. Mol Cell Biol. 1993;13:2787–801 Available from: http://www.ncbi.nlm.nih.gov/pubmed/8474441.
Brenner S, Prösch S, Schenke-Layland K, Riese U, Gausmann U, Platzer C. cAMP-induced Interleukin-10 promoter activation depends on CCAAT/enhancer-binding protein expression and monocytic differentiation. J Biol Chem. 2003;278:5597–604 Available from: http://www.ncbi.nlm.nih.gov/pubmed/12493739.
Fernández N, Renedo M, García-Rodríguez C, Sánchez CM. Activation of monocytic cells through Fc gamma receptors induces the expression of macrophage-inflammatory protein (MIP)-1 alpha, MIP-1 beta, and RANTES. J Immunol. 2002;169:3321–8 Available from: http://www.ncbi.nlm.nih.gov/pubmed/12218153.
Calonge E, Alonso-Lobo JM, Escandón C, González N, Bermejo M, Santiago B, et al. c/EBPbeta is a major regulatory element driving transcriptional activation of the CXCL12 promoter. J Mol Biol . Elsevier B.V. 2010;396:463–72. https://doi.org/10.1016/j.jmb.2009.11.064.
Tsushima H, Okazaki K, Ishihara K, Ushijima T, Iwamoto Y. CCAAT/enhancer-binding protein β promotes receptor activator of nuclear factor-kappa-B ligand (RANKL) expression and osteoclast formation in the synovium in rheumatoid arthritis. Arthritis Res Ther. 2015;17:–31 Available from: http://www.ncbi.nlm.nih.gov/pubmed/25811130.
Simpson-Abelson MR, Hernandez-Mir G, Childs EE, Cruz JA, Poholek AC, Chattopadhyay A, et al. CCAAT/enhancer-binding protein β promotes pathogenesis of EAE. Cytokine . Elsevier Ltd. 2017;92:24–32. https://doi.org/10.1016/j.cyto.2017.01.005.
Screpanti I, Romani L, Musiani P, Modesti A, Fattori E, Lazzaro D, et al. Lymphoproliferative disorder and imbalanced T-helper response in C/EBP beta-deficient mice. EMBO J. 1995;14:1932–41 Available from: http://www.ncbi.nlm.nih.gov/pubmed/7744000.
Zahnow CA. CCAAT/enhancer binding proteins in normal mammary development and breast cancer. Breast Cancer Res . BioMed Central. 2002;4:113–21 Available from: http://www.ncbi.nlm.nih.gov/pubmed/12052253.
Grimm SL, Rosen JM. The Role of C/EBPβ in Mammary Gland Development and Breast Cancer. J Mammary Gland Biol Neoplasia. 2003;8:191–204 Available from: http://www.ncbi.nlm.nih.gov/pubmed/14635794.
Acknowledgements
These studies were supported by grant CA16303 from the National Cancer Institute. We would like to thank Dr. Sandy Grimm for generously providing us with the figure illustrating C/EBPß expression in the mammary gland throughout development. While we tried to be as comprehensive as possible, we apologize to those investigators whose work we were unable to cite due to space limitations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Spike, A.J., Rosen, J.M. C/EBPß Isoform Specific Gene Regulation: It’s a Lot more Complicated than you Think!. J Mammary Gland Biol Neoplasia 25, 1–12 (2020). https://doi.org/10.1007/s10911-020-09444-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-020-09444-5