Abstract
Purpose
Parasite infections may lead to mortalities in fish; therefore, destabilizing the biodiversity and ecosystem functions. Swamps such as the Lorwai Swamp are important water sources, and information on the parasite species infecting Oreochromis nilotocus baringoensis in the hot springs of Lorwai Swamp which have a distinct genetic makeup from their counterparts in Lake Baringo is lacking. The purpose of this study was to provide a knowledge base on the parasite species infecting O. niloticus baringoensis in these springs, facilitate their comparison with those in Lake Baringo and determine their relationship with selected water quality parameters.
Methods
347 fish were collected and standard parasitological procedures were used to examine the presence of parasites. Physico-chemical parameters were measured in situ and water samples were collected for chlorophyll-a determination and nutrient analyses in the laboratory using standard methods. Relationship between parasitic infections and selected water quality parameters was determined by PCA using SPSS version 22.
Results
Two parasite species were common in all sites: Cichlidogyrus sclerosus and Clinostomum sp. Some parasites correlated positively with some parameters; Amirthalingamia macracantha and Contracaecum sp. with nitrogen compounds. Others like Clinostomum sp. and Tylodelphys sp. correlated negatively with dissolved oxygen.
Conclusion
Results from this study showed that there were both positive and negative relationships between some water quality parameters and the prevalence of recovered parasites. O. niloticus baringoensis from Lake Baringo also recorded high parasite prevalence and this calls for sensitization of the public on the risks that may arise from the consumption of undercooked infected fish.

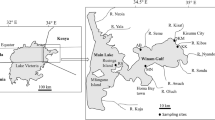
(Source: Modified from the Topographical map of Kenya scale 1:50,000) (Survey of Kenya)



Similar content being viewed by others
References
KMFRI (Kenya Marine and Fisheries Research Institute) (2017) Kenya’s aquaculture brief 2017: status, trends, challenges and future outlook. Kenya Marine and Fisheries Research Institute, Mombasa, p 12 (Accessed 14 May 2019)
Trewavas E (1983) Tilapiines fishes of the genera Sarotherodon, Oreochromis and Danakilia. British Museum (Natural History), London
Britton JR, Ng’eno JBK, Lugonzo J, Harper D (2006) Can an introduced, non-indigenous species save the fisheries of Lakes Baringo and Naivasha, Kenya? In: Odada EO, Olago DO, Ochola W, Ntiba M, Wandiga S, Gichuki N, Oyieke H (eds) Proceedings of the XI World Lake Conference, Nairobi, Kenya, vol. II. ILEC, Tokyo, pp 568–572
Odada EO, Onyando JO, Aloo PA (2006) Lake Baringo: Addressing threatened biodiversity and livelihoods. Lakes Reserv 11:1–13
Omondi R, Ojwang W, Olilo C, Mugo J, Agembe S, Ojuok JE (2016) Lakes Baringo and Naivasha: Endorheic freshwater lakes of the Rift Valley, Kenya. In: Finlayson CM, (eds) et al. The Wetland book. Springer Science+ Business Media, Dordrecht. https://doi.org/10.1007/978-94-007-6173-5_133-1
Nyingi D, De-Vos L, Aman R, Agnèse J-F (2009) Genetic characterization of an unknown and endangered native population of the Nile Tilapia Oreochromis niloticus (Linnaeus, 1758) (Cichlidae; Teleostei) in the Loboi Swamp (Kenya). Aquaculture 297:57–63
Paperna I (1996) Parasites, infections and diseases of fish in Africa. An update. FAO/CIFA Tech Paper 31:130–199
Younis AE, Saad AI, Rabei JM (2017) The occurrence of Contracaecum sp. larvae (Nematoda: Anisakidae) in four teleostean species from Lake Nasser, Egypt: morphological and molecular studies. J Basic Appl Zool 78:9
Chapman JM, Marcogliese DJ, Suski CD, Cooke SJ (2015) Variation in parasite communities and health indices of juvenile Lepomis gibbosus across a gradient of watershed land-use and habitat quality. Ecol Ind 57:564–572
Lafferty KD (1997) Environmental parasitology: what can parasites tell us about human impact on the environment? Parasitol Today 13:251–254
Marcogliese DJ (2005) Parasites of the superorganism: are they indicators of ecosystem health? Int J Parasitol 35:705–706
Blanar CA, Marcogliese DJ, Couillard CM (2011) Natural and anthropogenic factors shape metazoan parasite comminuty structure in mummichog (Fundulus hereroclitus) from two estuaries in New Brunswick, Canada. Folia Parasitol 58:240–248
Marcogliese DJ, Pietrock M (2011) combined effects of parasites and contaminants on animal health: parasites do matter. Trends Parasitol 27:123–130
Lafferty KD, Kuris AM (1999) How environmental stress affects the impacts of parasites. Limnol Oceanogr 44:925–931
Lazzaro BP, Little TJ (2009) Immunity in a variable world. Philos Trans R Soc Lond B 364:15–26
Wolinska J, King KC (2009) Environment can alter selection in host–parasite interactions. Trends Parasitol 25:236–244
Vonlanthen P, Bittner D, Hudson AG, Young KA, Müller R, Lundsgaard-Hansen B, Roy D, Di Piazza S, Largiader CR, Seehausen O (2012) Eutrophication causes speciation reversal in whitefish adaptive radiations. Nature 482:357–363
Condamine FL, Rolland J, Morlon H (2013) Macro evolutionary perspectives to environmental change. Ecol Lett 16:72–85
Smith TB, Kinnison MT, Strauss SY, Fuller TL, Carroll SP (2014) Prescriptive evolution to conserve and manage biodiversity. Annu Rev Ecol Evol Syst 45:1–22
Schmid-Hempel P (2003) Variation in immune defence as a question of evolutionary ecology. Proc R Soc B 270:357–366
Kutzer MA, Armitage SAO (2016) Maximising host fitness in the face of parasites: a review of host tolerance. Zoology 119:245–257
Mostowy R, Engelstädter J (2011) The impact of environmental change on host–parasite coevolutionary dynamics. Proc R Soc B 278:2283–2292
Budria A, Candolin U (2014) How does human-induced environmental change influence host–parasite interactions? Parasitology 141:462–474
Buser CC, Spaak P, Wolinska J (2012) Disease and pollution alter Daphnia taxonomic and clonal structure in experimental assemblages. Freshw Biol 57:1865–1874
Scharsack JP, Schweyen H, Schmidt AM, Dittmar J, Reusch TBH, Kurtz J (2012) Population genetic dynamics of three-spined sticklebacks (Gasterosteus aculeatus) in anthropogenic altered habitats. Ecol Evolut 2:1122–1143
IPCC (2014) Climate Change 2014: Synthesis Report Contributions of Working Groups I, II and III to the Fifth Assessment Report of the IPCC. Edited by Core Writing Team, Pachauri RK, Meyer LA Intergovernmental Panel on Climate Change, Geneva
Ricciardi A, Rasmussen JB (1999) Extinction rates of North American freshwater fauna. Conserv Biol 13:1220–1222
Williams HH, Jones A (1994) Parasitic worms of fish. Taylor & Francis Ltd., London
Marcogliese DJ (2004) Parasites: small players with crucial roles in the ecological theater. EcoHealth 1:151–164
Hedrick RP (1998) Relationships of the host, pathogen and environment: implications for diseases of cultured and wild fish populations. J Aquat Anim Health 10:107–111
Hossain MD, Hossain MK, Rahman MH (2007) Water quality parameters and incidences of fish diseases in some water bodies in Natore, Bangladesh. J Life Earth Sci 2(2):27–30
Bagge A, Valtonen ET (1996) Experimental study on the influence of paper and pulp mill effluent on the gill parasite communities of roach (Rutilus rutilus). Parasitology 112:499–508
Zargar UR, Chishti MZ, Yousuf AR, Fayaz A (2012) Infection level of monogenean gill parasite, Diplozoon kashmirensis (Monogenea, Polyopisthocotylea) in the Crucian carp, Carassius carassius from lake ecosystems of an altered water quality: what factors do have an impact on the Diplozoon infection? Vet Parasitol 189:218–226
Garcia F, Fujimoto RY, Martins ML, Moraes FR (2009) Protozoan parasites of Xiphophorus spp. (Poeciliidae) and their relation with water characteristics. Arq Bras Med Vet Zootec 61(1):156–162
Thomas CD, Cameron A, Green RE, Bakkenes M, Beaumont LJ, Collingham YC, Erasmus BFN, De Siqueira MF, Grainger A, Hannah L, Hughes L, Huntley B, Van Jaarsveld AS, Midgley GF, Miles L, Ortega-Huerta MA, Peterson AT, Townsend Peterson A, Phillips OL, Williams SE (2004) Extinction risk from climate change. Nature 427:145–148
Larsen MH, Mouritsen KN (2014) Temperature–parasitism synergy alters intertidal soft-bottom community structure. J Exp Mar Biol Ecol 460:109–119
Kutz SJ, Hoberg EP, Polley L, Jenkins EJ (2005) Global warming is changing the dynamics of Arctic host–parasite systems. Proc R Soc B 272:2571–2576
Scharsack JP, Franke F, Erin NI, Kuske A, Buescher J, Stolz H, Samonte IE, Kurtz J, Kalbe M (2016) Effects of environmental variation on host–parasite interaction in three-spined sticklebacks (Gasterosteus aculeatus). Zoology 119:287–296
MacNab V, Barber I (2012) Some (worms) like it hot: fish parasites grow faster in warmer water, and alter host thermal preferences. Glob Change Biol 18:1540–1548
Mateos-Gonzalez F, Sundström LF, Schmid M, Björklund M (2015) Rapid evolution of parasite resistance in a warmer environment: insights from a large-scale field experiment. PLoS ONE 10:e0128860
Yanong RPE (2002) Nematode (Roundworm) infections in fish. Institute of Food and Agricultural Sciences. Fisheries and Aquatic Sciences Department, University of Florida/IFAS Extension. http.//edis.Ifas.ufl.edu. Accessed 13 June 2019.
Marcogliese DJ, Cone DK (1997) Food webs: a plea for parasites. Trends Ecol Evol 13:320–325
Britton JR, Jackson MC, Harper DM (2009) Ligula intestinalis (Cestoda: Diphyllobothriidae) in Kenya: a field investigation into host specificity and behavioural alterations. Parasitology 136:1367–1373
Gumpinger P (2016) Parasites Species Richness of fish from Lake Baringo, Kenya. Masters Thesis, Universität für Bodenkultur Wien
Ashley GM, Goman MF, Hover CV, Owen RB, Renaut RW, Muasya AM (2002) Artesian blister wetlands, a perennial water resource in the semi-arid rift valley of East Africa. Wetlands 22(4):686–695
Ashley GM, Maitima MM, Muasya AM, Owen RB, Driese SG, Hover VC, Renaut RW, Goman MF, Mathias S, Blatt SH (2004) Sedimentation and recent history of a freshwater wetland in a semi-arid environment: Loboi Marsh, Kenya, East Africa. Sedimentology 51:1301–1321
Muasya AM, Hover VC, Ashley GM, Owen RB, Goman MF, Kimeli M (2004) Diversity and distribution of macrophytes in a freshwater wetland, Loboi Swamp (Rift valley) Kenya. J E Afr Nat Hist 93:39–47
Gitau PN, Verschuren D (2016) Sedimentological and paleoecological research techniques, with applications to the environmental history of Loboi Swamp, Kenya. J Earth Sci Clim Change 7(5):351
Omondi R, Yasindi AW, Magana AM (2013) Food and feeding habits of three main fish species in Lake Baringo. Kenya J Environ Microbiol 1(1):129–135
Onindo CO, Mwangi WI (2012) Thermodynamic modelling of the equilibria in Lake Baringo, Kenya. J Agric Sci Technol 14(2):1–10
APHA (American Public Health Association) (2004) Standard method for the examination of water and wastewater, 21st edn. America Water Works Association and Water Control Federation, Washington
Koroleff F (1999) Simultaneous oxidation of nitrogen and phosphorus compounds by persulfate. In: Grasshoff K, Kremling K, Ehrhardt M (eds) Methods of Seawater Analysis, Third, completely revised and extended edition. WILEY-VCH Publishers, Weinheim, pp 204–206
Schäperclaus W (1990) Fischkrankheiten. Akademie Verlag, Berlin
Lagler KF (1970) Capture, sampling and examination of fishes. In: Ricker WE (ed) Methods of assessment of fish production in freshwaters: IBP Handbook 3. Blackwell Scientific Publications, Oxford, pp 7–45
Kuchta R, Burianova A, Jirku M, Chambrier A, Oros M, Brabec J, Scholz T (2012) Botriocephalidean tapeworms (Cestoda) of freshwater fish in Africa, including erection of Kirstenella n. gen. and description of Tetracampos martinae n. sp. Zootaxa 3309:1–35
Paperna I (1980) Parasites, infections and diseases of fish in Africa. FAO/CIFA Tech Paper 7:216
Bush AO, Lafferty KD, Lotz JM, Shostak AWP (1997) Parasitology meets ecology on its own terms: Margolis, et al. Revisited. J Parasitol 83:575–583
Jirsa F, Konecny R, Frank C, Sures B (2011) The parasite community of the nase Chondrostoma nasus (L. 1758) from Austrian rivers. J Helminthol 85:255–262
Öǧüt H, Palm HW (2005) Seasonal dynamics of Trichodina spp. on whiting (Merlangius melangus) in relation to organic pollution on the eastern Black Sea coast of Turkey. Parasitol Res 96:149–153
Pozza A, Lima F, Haas ML, Lehmann-Albornoz PC (2018) Clinostomidae sp. (Digenea: Clinostomidae) and Ascocotyle sp. (Digenea: Heterophyidae): Metacercariae with zoonotic potential in fishes from Tramandaí river basin. Southern Brazil Bol Inst Pesca 44(1):105–109
Otachi EO (2009) Studies on occurrence of protozoan and helminth parasite in Nile tilapia (Oreochromis niloticus L.) from Central and Eastern provinces, Kenya. Masters Thesis, Egerton University, Kenya
Otachi EO, Locke SA, Jirsa F, Fellner-Frank C, Marcogliese DJ (2014) Morphometric and molecular analyses of Tylodelphys sp. metacercariae (Digenea: Diplostomidae) from the vitreous humour of four fish species from Lake Naivasha. Kenya. J Helminthol 89(04):404–414
Florio D, Gustinelli A, Caffara M, Turci F, Quaglio F, Konecny R, Nikowitz T, Wathuta ME, Magana A, Otachi EO, Matolla KG, Warugu WH, Liti D, Mbaluka R, Thiga B, Munguti J, Akoll P, Mwanja W, Asaminew K, Tadesse Z, Fioravanti ML (2009) Veterinary and public health aspects in tilapia (Oreochromis niloticus niloticus) aquaculture in Kenya, Uganda and Ethiopia. Ittiopatologia 6:51–93
Baker SC, Frank JB (1985) Effects of black-spot disease on the condition of stonerollers Campostoma anomalum. Am Midl Nat J 114(1):198–199
Rindoria NM, Mungai LK, Yasindi AW, Otachi EO (2016) Gill monogeneans of Oreochromis niloticus (Linnaeus, 1758) and Oreochromis leucostictus (Trewavas, 1933) in Lake Naivasha, Kenya. Parasitol Res 115:1501–1508
Zander CD (1998) Ecology of host parasite relationships in the Baltic Sea. Naturwissenschaften 85:426–436
Paperna I (1974) Larval Contracaecum in the pericardium of fishes from East African lakes. Proc Helminthol Soc Wash 41:252
Malvestuto SP, Ogambo-Ongoma A (1978) Observation of the Infection of Tilapia leucosticte (Pisces: Cichlidae) with Contracaecum (Nematoda: Heterocheilidae) in Lake Naivasha, Kenya. J Parasitol 64:383–384
Aloo PA (2002) A comparative study of helminth parasites from the fish Tilapia zillii and Oreochromis leucostictus in Lake Naivasha and Oloidien Bay, Kenya. J Helminthol 76:95–102
Steckler N, Yanong RPE (2012) Argulus (Fish Louse) infections in fish. Institute of food and agricultural sciences. Fisheries and Aquatic Sciences Department, University of Florida/IFAS Extension. http.//edis.Ifas.ufl.edu. Accessed 13 June 2019
Levsen A, Svanesik CS, Cipriani P, Mattiucci C, Gay M, Hatie LC, Bušelić I, Mladineo I, Karl H, Ostermeyer U, Buchmann K, Hӧjgaard DP, González AF, Pascual S, Pierce GJ (2017) A survey of Zoonotic nematodes of commercial key fish species from major European fishing grounds–Introducing the FP7 parasite exposure assessment study. Fish Res 202:4–21
Khan RA (2012) Host-Parasite Interactions in some Fish Species. J Parasitol Res 2012:1–7
Lõhmus M, Björklund M (2015) Climate change: what will it do to fish-parasite interactions? Biol J Lin Soc 116:397–411
Sreenivasan A (1976) Limnological studies of and primary production in temple pond ecosystems. Hydrobiologia 48:117–123
Bhatnagar A, Devi P (2013) Water quality guidelines for the management of pond fish culture. Int J Environ Sci 3(6):1980–2009
Ngugi CC, Bowman JR, Omolo BO (2007) A new guide to fish farming in Kenya. Aquaculture CRSP, Oregon, USA
Bauer ON (1961) Parasitic diseases of cultured fishes and methods of their prevention and treatment. In: Dogiel VA, Petrushevski GK, Polyanski YI (eds) Parasitology of fishes. Oliver and Boyd, Edinburgh, pp 265–298
Paredes-Trujillo A, Velázquez-Abunader I, Torres-Irineo E, Romero D, Vidal-Martínez VM (2016) Geographical distribution of protozoan and metazoan parasites of farmed Nile tilapia Oreochromis niloticus (L.) (Perciformes: Cichlidae) in Yucatán, México. Parasite Vector 9:66–81
Sures B (2004) Environmental parasitology: relevancy of parasites in monitoring environmental pollution. Trends Parasitol 20:170–177
Wetzel RG (2001) Limnology: lake and river ecosystems. Elservier Academic Press, Netherlands
Koyun M (2011) The effect of water temperature on Argulus foliaceus L. 1758 (Crustacea; Branchiura) on different fish species. Not Sci Biol 3(2):16–19
Acknowledgements
Special thanks go to Mr. Arthur Adamba Kafuna and the late Ms. Naomi Nyambura Kamau for funding this research. We acknowledge the Department of Biological Sciences of Egerton University for allowing us use their laboratory and equipment, the committee of the Lorwai Swamp Conservancy for allowing us to carry out this study in the hot springs of Lorwai Swamp and the technical assistance of Mr. Felix Muvea and Mr. Titus Rono in the field and Mr. Lewis Mungai in the laboratory. We also acknowledge the National Commission for Science, Technology and Innovation (NACOSTI) for granting us the permission to carry out this study (Permit No: NACOSTI/P/19/73132/27709).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or non-for-profit sectors, but was funded personally by Mr. Arthur Adamba Kafuna and the late Ms. Naomi Nyambura Kamau.
This research complied with the Egerton University Ethics Review Committee ethical standards and guidelines.
Author information
Authors and Affiliations
Contributions
SWKA carried out the field and laboratory work and wrote the first draft of the manuscript. EOO and GOO made critical suggestions towards the improvement of the manuscript and critically reviewed it. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Adamba, S.W.K., Otachi, E.O. & Ong’ondo, G.O. Parasite Communities of Oreochromis niloticus baringoensis (Trewavas, 1983) in Relation to Selected Water Quality Parameters in the Springs of Lorwai Swamp and Lake Baringo, Kenya. Acta Parasit. 65, 441–451 (2020). https://doi.org/10.2478/s11686-020-00178-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11686-020-00178-2