Abstract
Ultrafiltration/diafiltration (UF/DF) plays an important role in the manufacturing of biopharmaceuticals. Monitoring critical process parameters and quality attributes by process analytical technology (PAT) during those steps can facilitate process development and assure consistent quality in production processes. In this study, a lab-scale cross-flow filtration (CFF) device was equipped with a variable pathlength (VP) ultraviolet and visible (UV/Vis) spectrometer, a light scattering photometer, and a liquid density sensor (microLDS). Based on the measured signals, the protein concentration, buffer exchange, apparent molecular weight, and hydrodynamic radius were monitored. The setup was tested in three case studies. First, lysozyme was used in an UF/DF run to show the comparability of on-line and off-line measurements. The corresponding correlation coefficients exceeded 0.97. Next, urea-induced changes in protein size of glucose oxidase (GOx) were monitored during two DF steps. Here, correlation coefficients were ≥ 0.92 for static light scattering (SLS) and dynamic light scattering (DLS). The correlation coefficient for the protein concentration was 0.82, possibly due to time-dependent protein precipitation. Finally, a case study was conducted with a monoclonal antibody (mAb) to show the full potential of this setup. Again, off-line and on-line measurements were in good agreement with all correlation coefficients exceeding 0.92. The protein concentration could be monitored in-line in a large range from 3 to 120 g L− 1. A buffer-dependent increase in apparent molecular weight of the mAb was observed during DF, providing interesting supplemental information for process development and stability assessment. In summary, the developed setup provides a powerful testing system for evaluating different UF/DF processes and may be a good starting point to develop process control strategies.

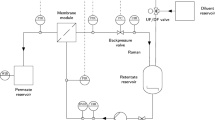
Piping and instrumentation diagram of the experimental setup and data generated by the different sensors. A VP UV/Vis spectrometer (FlowVPE, yellow) measures the protein concentration. From the data of the light scattering photometer (Zetasizer, green) in the on-line measurement loop, the apparant molecular weight and z-average are calculated. The density sensor (microLDS) measures density and viscosity of the fluid in the on-line loop









Similar content being viewed by others
References
Bakeev KA. Process analytical technology: spectroscopic tools and implementation strategies for the chemical and pharmaceutical industries. Hoboken: Wiley; 2010.
Food and Drug Administration (FDA). 2004. Guidance for industry, PAT—a framework for innovative pharmaceutical development manufacturing and quality assurance.
Hinz DC. Process analytical technologies in the pharmaceutical industry: the FDA’s PAT initiative. Anal Bioanal Chem 2006;384(5):1036–1042.
Roch P, Mandenius C-F. On-line monitoring of downstream bioprocesses. Current Opinion in Chemical Engineering 2016;14:112–120.
Rathore AS, Kapoor G. Application of process analytical technology for downstream purification of biotherapeutics. J Chem Technol Biotechnol 2015;90(2):228–236.
Rüdt M., Briskot T, Hubbuch J. Advances in downstream processing of biologics—spectroscopy: an emerging process analytical technology. J Chromatogr A 2017;1490:2–9.
Brestrich N, Briskot T, Osberghaus A, Hubbuch J. A tool for selective inline quantification of co-eluting proteins in chromatography using spectral analysis and partial least squares regression. Biotechnol Bioeng 2014;111(7): 1365–1373.
Brestrich N, Sanden A, Kraft A, McCann K, Bertolini J, Hubbuch J. Advances in inline quantification of co-eluting proteins in chromatography: process-data-based model calibration and application towards real-life separation issues. Biotechnol Bioeng 2015;112(7):1406–1416.
Rüdt M., Brestrich N, Rolinger L, Hubbuch J. Real-time monitoring and control of the load phase of a protein a capture step. Biotechnol Bioeng 2017;114(2):368–373.
Fahrner RL, Blank GS. Real-time control of antibody loading during protein a affinity chromatography using an on-line assay. J Chromatogr A 1999;849(1):191–196.
Fahrner RL, Lester PM, Blank GS, Reifsnyder DH. Real-time control of purified product collection during chromatography of recombinant human insulin-like growth factor-i using an on-line assay. J Chromatogr A 1998;827 (1):37–43.
Fahrner RL, Blank GS. Real-time monitoring of recombinant antibody breakthrough during protein a affinity chromatography. Biotechnol Appl Biochem 1999;29(2):109–112.
Gottschalk U. Process scale purification of antibodies. Hoboken: Wiley; 2017.
Jagschies G, Lindskog E, Lacki K, Galliher PM. Biopharmaceutical processing: development, design, and implementation of manufacturing processes. Amsterdam: Elsevier; 2018.
Rathore AS, Sharma A, Chilin D. 2006. Applying process analytical technology to biotech unit operations. Biopharm International 19 (8).
Donnan FG. Theorie der Membrangleichgewichte und Membranpotentiale bei Vorhandensein von nicht dialysierenden Elektrolyten. Ein Beitrag zur physikalisch-chemischen Physiologie. Zeitschrift Für Elektrochemie Und Angewandte Physikalische Chemie 1911;17(14):572–581.
Arunkumar A, Zhang J, Singh N, Ghose S, Li ZJ. Ultrafiltration behavior of partially retained proteins and completely retained proteins using equally-staged single pass tangential flow filtration membranes. Biotechnol Prog 2018;34(5):1137–1148.
Rüdt M., Vormittag P, Hillebrandt N, Hubbuch J. Process monitoring of virus-like particle reassembly by diafiltration with UV/Vis spectroscopy and light scattering. Biotechnol Bioeng 2019;116(6):1366–1379.
Savitzky A, Golay MJ. Smoothing and differentiation of data by simplified least squares procedures. Anal Chem 1964;36(8):1627–1639.
Jiskoot W., Crommelin D., (eds). 2005. Methods for structural analysis of protein pharmaceuticals. Arlington: American Association of Pharmaceutical Scientists.
Mach H, Middaugh CR. Simultaneous monitoring of the environment of tryptophan, tyrosine, and phenylalanine residues in proteins by near-ultraviolet second-derivative spectroscopy. Analytical Biochemistry 1994; 222:323–331. https://doi.org/10.1006/abio.1994.1499.
Ragone R, Colonna G, Balestrieri C, Servillo L, Irace G. Determination of tyrosine exposure in proteins by second-derivative spectroscopy. Biochemistry 1984;23(8):1871–1875.
Einstein A. Über die von der molekularkinetischen theorie der wärme geforderte bewegung von in ruhenden flüssigkeiten suspendierten teilchen. Annalen Der Physik 1905;17(4):549–560.
Godfrin PD, Hudson SD, Hong K, Porcar L, Falus P, Wagner NJ, Liu Y. Short-time glassy dynamics in viscous protein solutions with competing interactions. Phys Rev Lett 2015;115(22):228302.
Godfrin PD, Zarraga IE, Zarzar J, Porcar L, Falus P, Wagner NJ, Liu Y. Effect of hierarchical cluster formation on the viscosity of concentrated monoclonal antibody formulations studied by neutron scattering. J Phys Chem B 2016;120(2):278–291.
Lebowitz J, Lewis MS, Schuck P. Modern analytical ultracentrifugation in protein science: a tutorial review. Protein Sci 2002;11(9):2067–2079.
Lemmon E, McLinden M, Friend D. 2005. NIST chemistry webbook, NIST standard reference database number 69, National Institute of Standards and Technology, Gaithersburg MD-US, Ch. Thermophysical Properties of Fluid Systems. https://doi.org/10.18434/T4D303.
Koppel DE. Analysis of macromolecular polydispersity in intensity correlation spectroscopy: the method of cumulants. J Chem Phys 1972;57(11):4814–4820. arXiv:1011.1669v3. https://doi.org/10.1063/1.1678153.
Thomas JC. The determination of log normal particle size distributions by dynamic light scattering. J Colloid Interface Sci 1987;117(1):187–192.
Sun Z, Deluca T, Mattison K. The size and rheology characterization of concentrated emulsions. Am Lab 2005;37(12):8.
Malvern. 2017. Application of dynamic light scattering (DLS) to protein therapeutic formulations: principles, measurements and analysis–2. concentration effects and particle interactions, Tech. rep., Grovewood Road, Malvern, Worcestershire, UK.
Dubin SB, Lunacek JH, Benedek GB. Observation of the spectrum of light scattered by solutions of biological macromolecules. Proc Natl Acad Sci 1967;57(5):1164–1171.
Forzani ES, Otero M, Pérez M. A., Teijelo ML, Calvo EJ. The structure of layer-by-layer self-assembled glucose oxidase and Os (Bpy) 2CLPyCH2NH- poly (allylamine) multilayers: ellipsometric and quartz crystal microbalance studies. Langmuir 2002;18(10):4020–4029.
Zoldák G, Zubrik A, Musatov A, Stupák M, Sedlák E. Irreversible thermal denaturation of glucose oxidase from Aspergillus niger is the transition to the denatured state with residual structure. J Biol Chem 2004;279 (46):47601–47609.
Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discovery 2014;13(9):655.
Yadav S, Shire SJ, Kalonia DS. Factors affecting the viscosity in high concentration solutions of different monoclonal antibodies. J Pharm Sci 2010;99(12):4812–4829.
Lewis E, Qi W, Kidder L, Amin S, Kenyon S, Blake S. Combined dynamic light scattering and raman spectroscopy approach for characterizing the aggregation of therapeutic proteins. Molecules 2014;19(12): 20888–20905.
Mahler H-C, Jiskoot W. Analysis of aggregates and particles in protein pharmaceuticals. Hoboken: Wiley; 2011.
Arakawa T, Philo JS, Ejima D, Tsumoto K, Arisaka F. Aggregation analysis of therapeutic proteins, part 2. Bioprocess International 2007;5(4):36–47.
O’Malley JJ, Weaver JL. Subunit structure of glucose oxidase from Aspergillus niger. Biochemistry 1972; 11(19):3527–3532.
Swoboda BE, Massey V. On the reaction of the glucose oxidase from Aspergillus niger with bisulfite. J Biol Chem 1966;241(14):3409–3416.
Gouda M, Thakur M, Karanth N. Reversible denaturation behavior of immobilized glucose oxidase. Appl Biochem Biotechnol 2002;102(1-6):471–480.
Liu J, Lu J, Zhao X, Lu J, Cui Z. Separation of glucose oxidase and catalase using ultrafiltration with 300-kDa polyethersulfone membranes. J Membr Sci 2007;299(1-2):222–228.
Wilkins DK, Grimshaw SB, Receveur V, Dobson CM, Jones JA, Smith LJ. Hydrodynamic radii of native and denatured proteins measured by pulse field gradient NMR techniques. Biochemistry 1999;38(50): 16424–16431.
Liu J, Nguyen MD, Andya JD, Shire SJ. Reversible self-association increases the viscosity of a concentrated monoclonal antibody in aqueous solution. J Pharm Sci 2005;94(9):1928–1940.
Philo J. A critical review of methods for size characterization of non-particulate protein aggregates. Curr Pharm Biotechnol 2009;10(4):359–372.
Ahrer K, Buchacher A, Iberer G, Josic D, Jungbauer A. Analysis of aggregates of human immunoglobulin g using size-exclusion chromatography, static and dynamic light scattering. J Chromatogr A 2003; 1009(1-2):89–96.
Philo JS. Is any measurement method optimal for all aggregate sizes and types?. The Aaps Journal 2006;8(3): E564–E571.
Bam NB, Cleland JL, Yang J, Manning MC, Carpenter JF, Kelley RF, Randolph TW. Tween protects recombinant human growth hormone against agitation-induced damage via hydrophobic interactions. J Pharm Sci 1998;87(12):1554–1559. https://doi.org/10.1021/js980175v.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection Advances in Process Analytics and Control Technology with guest editor Christoph Herwig.
Laura Rolinger and Matthias Rüdt contributed equally to this work.
Appendix: Debye plots
Appendix: Debye plots
Figure 10 shows the regressions curves for the calculation of the second virial coefficient and Table 2 shows the obtained values. Every measurement of the calibration points consists of the average of three runs. The standard deviation of those three runs is not shown in the graph because they were consistently less than 1%. As mentioned in the “Off-line analytics by light scattering” section, five concentration series were measured for GOx and mAb to obtain different A2 for each buffer composition. Due to the instability of GOx in urea buffer and the relatively low concentration, buffer related changes in the A2 were neglected. Instead the A2 of GOx in the feed buffer was used for the whole process. For the mAb concentrations of up to 120 g L− 1 were reached during the second UF. Therefore, an extra A2 for the DF buffer was used because with higher concentrations the A2 value becomes more important for the calculation of the apparent molecular weight.
For the calculation of the second virial coefficient A2, concentration series for lysozyme (a), GOx (b), and mAb (c) were measured by SLS (circles). The gradient of the regression line was divided by two and used as A2. For lysozyme and GOx, the concentration series was only measured with feed buffer (blue). For the mAb, a concentration in both the feed (orange) and DF buffer (blue) was measured
Rights and permissions
About this article
Cite this article
Rolinger, L., Rüdt, M., Diehm, J. et al. Multi-attribute PAT for UF/DF of Proteins—Monitoring Concentration, particle sizes, and Buffer Exchange. Anal Bioanal Chem 412, 2123–2136 (2020). https://doi.org/10.1007/s00216-019-02318-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02318-8