Abstract
Synthesis of bioactive heterocyclic compounds having effective biological activity is an essential research area for wide-ranging applications. In this study, a conventional methodology has been developed for the synthesis of a series of new 3-mercapto-1,2,4-triazole derivatives 4a–f. The purity and structure of the synthesized molecules were confirmed by 1H NMR, 13C NMR and elemental analysis. In addition, the prepared compounds were screened for their anti-proliferative activity against three human cancer cell lines including A549 (lung cancer), MCF7 (breast cancer) and SKOV3 (ovarian cancer) using MTT reduction assay. All the tested compounds demonstrated remarkable cytotoxic activity with IC50 values ranging from 3.02 to 15.37 µM. The heterocyclic compound bearing 3,4,5-trimethoxy moiety was found to be the most effective among the series displaying an IC50 of 3.02 µM specifically against the ovarian carcinoma cancer cell line (SKOV3). Moreover, Annexin V-FITC/propidium iodide staining assay indicated that this compound can induce apoptosis in SKOV3 cells. Furthermore, cell cycle assay showed a significant cell cycle arrest at the G2/M phase in a dose-dependent manner for this compound. The molecular docking results was showed binding modes of potent compound 4d perfectly corroborated the suggestion of binding to the colchicine site. The entire results conclude that 3-mercapto-1,2,4-triazole derivatives can be synthesized by a green method for biological and pharmacological applications.
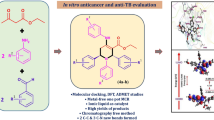
Graphic abstract
New analogs of 3-mercapto-1,2,4-triazole potential derivatives for anti-proliferative activity were synthesized. Cytotoxic activity of all synthesized compounds was evaluated against tree human cancer cell lines: lung (A549), breast (MCF7) and ovarian (SKOV3).







Similar content being viewed by others
References
Palaniraja J, Roopan SM (2015) Iodine-mediated synthesis of indazolo-quinazolinones via a multi-component reaction. RSC Adv 5:8640–8646
Roopan SM, Bharathi A, Palaniraja J, Anand K, Gengan RM (2015) Unexpected regiospecific Michael addition product: synthesis of 5,6-dihydrobenzo[1,7]phenanthrolines. RSC Adv 5:38640–38645
Roopan SM, Khan FRN, Mandal BK (2010) Fe nano particles mediated C–N bond forming reaction: regio-selective synthesis of 3-[(2-chloroquinolin-3-yl)methyl]pyrimidin-4(3H)ones. Tetrahedron Lett 51:2309–2311
Anderson DK, Deuwer DL, Sirkorski JA (1995) Syntheses of new 2-hydroxythiazol- 5-yl and 3-hydroxy-1,2,4-triazol-ylphosphonic acids as potential cyclic spatial mimics of glyphosate. J Heterocycl Chem 32:893–898
Collin X, Sauleau A, Coulon J (2003) 1,2,4-Triazolomercapto and aminonitriles as potent antifungal agents. Bioorg Med Chem Lett 13:2601–2605
Heindel ND, Reid JR (1980) 4-Amino-3-mercapto-4H-1,2,4-triazoles and propargyl aldehydes: a new route to 3-R-8-aryl-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazepines. J Heterocycl Chem 17:1087–1088
Holla BS, Kalluraya B, Sridhar KR, Drake E, Thomas LM, Bhandary KK, Levine MS (1994) Synthesis, structural characterization, crystallographic analysis and antibacterial properties of some nitrofuryl triazolo[3,4-b]-1,3,4-thiadiazines. Eur J Med Chem 29:301–308
Mathew V, Keshavayya J, Vidya VP, Acharya Reddy BM (2006) Heterocyclic system containing bridgehead nitrogen atom: synthesis and pharmacological activities of some substituted 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles. Eur J Med Chem 41:1048–1058
Shehy MF, Abu-Hashem A, El-Telbani EM (2010) Synthesis of 3-((2,4-dichlorophenoxy)methyl)-1,2,4-triazolo(thiadiazoles and thiadiazines) as anti-inflammatory and molluscicidal agents. Eur J Med Chem 45:1906–1911
Heeres J, Backx LJ (1984) Antimycotic azoles. 7. Synthesis and antifungal properties of a series of novel triazol-3-ones. J Med Chem 27:894–900
Wu J, Yu W, Fu L, He W, Wang Y, Chai B, Song C, Chang J (2013) Design, synthesis, and biological evaluation of new 2′-deoxy-2′-fluoro-4′-triazole cytidine nucleosides as potent antiviral agents. Eur J Med Chem 63:739–745
Palekar VS, Damle AJ, Shukla SR (2009) Synthesis and antibacterial activity of some novel bis-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles and bis-4-thiazolidinone derivatives from terephthalic dihydrazide. Eur J Med Chem 44:5112–5116
Siddiqui AA, Mishra R, Shaharyar M, Husain A, Rashid M, Pal P (2011) Triazole incorporated pyridazinones as a new class of antihypertensive agents: design, synthesis and in vivo screening. Bioorg Med Chem Lett 21:1023–1026
Silva STD, Visbal G, Godinho JLP, Urbina JA, de Souza W, Rdrigues JCF (2018) In vitro antileishmanial activity of ravuconazole, a triazole antifungal drug, as a potential treatment for leishmaniasis. J Antimicrob Chemother 73:2360–2373
Fard JK, Hamzeiy H, Sattari M, Eftekhari A, Ahmadian E, Eghbal MA (2016) Triazole rizatriptan induces liver toxicity through lysosomal/mitochondrial dysfunction. Drug Res 66:470–478
Uemura SI, Kanbayashi T, Wakasa M, Satake M, Ito W, Shimizu K, Shioya T, Shimizu T, Nishino S (2015) Residual effects of zolpidem, triazolam, rilmazafone and placebo in healthy elderly subjects: a randomized double-blind study. Sleep Med 16:1395–1402
Coffen DL, Fryer RI (1974) US Patent, 3,849,434
Shiroki M, Tahara T, Araki K (1975) Japan Patent, 75,100,096
Lemke TL, Williams DA, Roche VF, Zito SW (2013) In: Lemke TL, Williams DA (eds) Foye’s principles of medicinal chemistry, 7th edn. Lippincott Williams and Wilkins, Philadelphia
Deason ME, Whitten KR (1999) US Patent, 5,962,725
Bell AS, Brown D, Terrett NK (1993) US Patent, 5,250,534
Rawlins AL, Woods GP (1952) US Patent, 2,589,211
Mavrova AT, Wesselinova D, Tsenov JA, Lubenov LA (2014) Synthesis and antiproliferative activity of some new thieno[2,3-d]pyrimidin-4(3H)-ones containing 1,2,4-triazole and 1,3,4-thiadiazole moiety. Eur J Med Chem 86:676–683
Gupta D, Jain DK (2015) Synthesis, antifungal and antibacterial activity of novel 1,2,4-triazole derivatives. J Adv Pharm Technol Res 6:141–146
Küçükgüzel ŞG, Çıkla-Süzgün P (2015) Recent advances bioactive 1,2,4-triazole-3-thiones. Eur J Med Chem 97:830–870
Clemons M, Coleman RE, Verma S (2004) Aromatase inhibitors in the adjuvant setting: bringing the gold to a standard? Cancer Treat Rev 30:325–332
Rajabi M, Hossaini Z, Khalilzadeh MA, Datta S, Halder M, Mousa SA (2015) Synthesis of a new class of furo[3,2-c]coumarins and its anticancer activity. J Photochem Photobiol, B 148:66–72
Khaleghi F, Jantan I, Din LB, Yaacob WA, Khalilzadeh MA, Bukhari SNA (2014) Immunomodulatory effects of 1-(6-hydroxy-2-isopropenyl-1-benzofuran-5-yl)-1-ethanone from Petasites hybridus and its synthesized benzoxazepine derivatives. J Nat Med 68:351–357
Tavakolinia F, Baghipour T, Khalilzadeh MA, Hossaini Z, Rajabi M (2012) Antiproliferative activity of novel thiopyran analogs on MCF-7 breast and HCT-15 colon cancer cells: synthesis, cytotoxicity, cell cycle analysis, and DNA-binding. Nucleic Acid Ther 22:265–270
Rajabi M, Khalilzadeh MA, Mehrzad J (2012) Antiproliferative activity of novel derivative of thiopyran on breast and colon cancer lines and DNA binding. DNA Cell Biol 31:128–134
Rajabi M, Khalilzadeh MA, Tavakolinia F, Signorelli P, Ghidoni R, Santaniello E (2012) Naphthalene-fused (a-Alkoxycarbonyl)methylene-g-butyrolactones: antiproliferative activity and binding to bovine serum albumin and DNA. DNA Cell Biol 31:783–789
Dalvie DK, Kalgutkar AS, Khojasteh-Bakht SC, Obach RS, O’Donnell JP (2002) Biotransformation reactions of five-membered aromatic heterocyclic rings. Chem Res Toxicol 15:269–299
Romagnoli R, Baraldi PG, Salvador MK, Prencipe F, Bertolasi V, Cancellieri M, Brancale A, Hamel E, Castagliuolo I, Consolaro F, Porcù E, Basso G, Viola G (2014) Synthesis, antimitotic and antivascular activity of 1-(3′,4′,5′-trimethoxybenzoyl)-3-arylamino-5-amino-1,2,4-triazoles. J Med Chem 57:6795–6808
van Meerloo J, Kaspers GJL, Cloos J (2011) Cell sensitivity assays: the MTT assay. Methods Mol Biol 731:237–245
Forli S, Huey R, Pique ME, Sanner MF, Goodsell DS, Olson AJ (2016) Computational protein-ligand docking and virtual drug screening with the AutoDock suite. Nat Protoc 11:905–919
Khan I, Ibrar A, Abbas N (2013) Triazolothiadiazoles and triazolothiadiazines-biologically attractive scaffolds. Eur J Med Chem 63:854–868
Kamel MM, Abdo NYM (2014) Synthesis of novel 1,2,4-triazoles, triazolothiadiazines and triazolothiadiazoles as potential anticancer agents. Eur J Med Chem 86:75–80
Tron GC, Pirali T, Sorba G, Pagliai F, Busacca S, Genazzani AA (2006) Medicinal chemistry of combretastatin A4: present and future directions. J Med Chem 49:3033–3044
Hsieh HP, Liou JP, Mahindroo N (2005) Pharmaceutical design of antimitotic agents based on combretastatins. Curr Pharm Des 11:1655–1677
Zhang B, Li YH, Liu Y, Chen YR, Pan E, You WW, Zhao LP (2015) Design, synthesis and biological evaluation of novel 1,2,4-triazolo [3,4-b][1,3,4] thiadiazines bearing furan and thiophene nucleus. Eur J Med Chem 103:335–342
El-Sherief HAM, Youssif BGM, Bukhari SNA, Abdel-Rahman MAM (2017) Novel 1,2,4-triazole derivatives as potential anticancer agents: design, synthesis, molecular docking and mechanistic studies. Bioorg Chem 17:30752–30756
Mioc M, Avram S, Bercean V, Kurunczi L, Ghiulai RM, Oprean C, Coricovac DE, Dehelean C, Mioc A, Porcarasu MB, Tatu C, Soica C (2018) Design, synthesis and biological activity evaluation of S-substituted 1H-5-mercapto-1,2,4-triazole derivatives as antiproliferative agents in colorectal cancer. Front Chem 6:1120–1126
Murty MSR, Ram KR, Rao RV, Yadav JS, Rao JV, Velatooru LR (2012) Synthesis of new S-alkylated-3-mercapto-1,2,4-triazole derivatives bearing cyclic amine moiety as potent anticancer agents. Lett Drug Des Discov 9:276–281
Zhu H, Zhang J, Xue N, Hu Y, Yang B, He Q (2010) Novel combretastatin A-4 derivative XN0502 induces cell cycle arrest and apoptosis in A549 cells. Invest New Drugs 28:493–501
Ducki S, Mackenzie G, Lawrence NJ, Snyder JP (2005) Quantitative structure activity relationship (5D-QSAR) study of combretastatin-like analogues as inhibitors of tubulin assembly. J Med Chem 48:457–465
Negi AS, Gautam Y, Alam S, Chanda D, Luqman S, Sarkar J, Khan F, Konwar R (2015) Natural antitubulin agents: importance of 3,4,5-trimethoxyphenyl fragment. Bioorg Med Chem 23:373–389
Lu Y, Chen J, Xiao M, Li W, Miller DD (2012) An overview of tubulin inhibitors that interact with the colchicine binding site. Pharm Res 29:2943–2971
Prota AE, Danel F, Bachmann F, Bargsten K, Buey RM, Pohlmann J, Reinelt S, Lane H (2014) Steinmetz MO the novel microtubule-destabilizing drug BAL27862 binds to the colchicine site of tubulin with distinct effects on microtubule organization. J Mol Biol 426:1848–1860
Kamal A, Srikanth PS, Vishnuvardhan MVPS, Bharath Kumar G, Suresh Babu K, Hussaini SMA, Kapure JS, Alarifi A (2016) Combretastatin linked 1,3,4-oxadiazole conjugates as a potent tubulin polymerization inhibitors. Bioorg Chem 65:126–136
Guggilapu SD, Guntuku L, Srinivasa Reddy T, Nagarsenkar A, Sigalapalli DK, Naidu VGM, Bhargava SK, Babu Bathini N (2017) Synthesis of thiazole linked indolyl-3-glyoxylamide derivatives as tubulin polymerization inhibitors. Eur J Med Chem 138:83–95
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghanaat, J., Khalilzadeh, M.A. & Zareyee, D. Molecular docking studies, biological evaluation and synthesis of novel 3-mercapto-1,2,4-triazole derivatives. Mol Divers 25, 223–232 (2021). https://doi.org/10.1007/s11030-020-10050-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-020-10050-0