Abstract
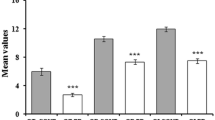
The pathophysiology of cervical dystonia is not completely understood. Current concepts of the pathophysiology propose that it is a network disorder involving the basal ganglia, cerebellum and sensorimotor cortex. These structures are primarily concerned with sensorimotor control but are also involved in non-motor functioning such as the processing of information related to the chemical senses. This overlap lets us hypothesize a link between cervical dystonia and altered sense of smell and taste. To prove this hypothesis and to contribute to the better understanding of cervical dystonia, we assessed olfactory and gustatory functioning in 40 adults with idiopathic cervical dystonia and 40 healthy controls. The Sniffin Sticks were used to assess odor threshold, discrimination and identification. Furthermore, the Taste Strips were applied to assess the combined taste score. Motor and non-motor deficits of cervical dystonia including neuropsychological and psychiatric alterations were assessed as cofactors for regression analyses. We found that cervical dystonia subjects had lower scores than healthy controls for odor threshold (5.8 ± 2.4 versus 8.0 ± 3.2; p = 0.001), odor identification (11.7 ± 2.3 versus 13.1 ± 1.3; p = 0.001) and the combined taste score (9.5 ± 2.2 versus 11.7 ± 2.7; p < 0.001), while no difference was found in odor discrimination (12.0 ± 2.5 versus 12.9 ± 1.8; p = 0.097). Regression analysis suggests that age is the main predictor for olfactory decline in subjects with cervical dystonia. Moreover, performance in the Montreal Cognitive Assessment is a predictor for gustatory decline in cervical dystonia subjects. Findings propose that cervical dystonia is associated with diminished olfactory and gustatory functioning.
Similar content being viewed by others
Introduction
Cervical dystonia (CD) is the most common form of adult onset focal dystonia (Albanese et al. 2013; ESDE 2000). Beside dystonia affecting the head, neck and shoulder muscles as well as action induced tremors, there are numerous non-motor deficits in CD (Kuyper et al. 2011). These include sensory abnormalities, such as pain (Tinazzi et al. 2019), sensory tricks (Sarasso et al. 2020), altered temporal and spatial discrimination (Junker et al. 2019; Conte et al. 2019) and impaired temperature detection threshold (Paracka et al. 2017). Furthermore, there are mild neuropsychological deficits (such as deficits of memory, verbal fluency and executive functioning) as well as psychiatric alterations (such as depression and anxiety) (Kuyper et al. 2011).
The pathophysiology of CD is not completely understood. Current concepts of its pathophysiology propose alterations in a network including the sensorimotor cortex, basal ganglia and cerebellum (Prudente et al. 2014; Quartarone and Hallett 2013; Shakkottai et al. 2017). While this network is primarily concerned with sensorimotor control (Quartarone and Hallett 2013), the basal ganglia and cerebellum are also important for non-motor functioning (Alexander et al. 1986; Bodranghien et al. 2016; Bostan et al. 2013; Gerwig et al. 2010; Eisinger et al. 2018; Saint-Cyr 2003; Strick et al. 2009; Weintraub and Zaghloul 2013). Additionally, there is evidence for the involvement of basal ganglia and cerebellum in the chemical senses. Tracing studies in animals revealed that basal ganglia receive input from both the primary gustatory cortex (which is primarily the insular cortex) (Fudge et al. 2005) and primary olfactory cortex (which includes olfactory tubercle) (Alexander et al. 1986; Eslinger et al. 1982; Russchen et al. 1985), whereas the cerebellum receives olfactory input via the ventral tegmental area from the piriform cortex (Ikai et al. 1992, 1994). Additionally, functional imaging studies in healthy human subjects showed activation of the cerebellum and the basal ganglia with olfactory tasks (Savic 2002). Furthermore, focal and degenerative cerebellar disease causes olfactory decline (Connelly et al. 2003; Mainland et al. 2005; Zobel et al. 2010). The sensorimotor cortex receives afferent projections from the central gustatory tracts (Heckmann et al. 2003; Mascioli et al. 2015). In addition, functional imaging showed activation of the sensorimotor cortex with gustatory stimulation of the tongue (Mascioli et al. 2015; Wistehube et al. 2018).
Based on the overlap between neuroanatomical regions involved in the pathophysiology of CD and processing of information related to the chemical senses, altered sense of smell and taste may be found in CD. In fact, few studies have explored the sense of smell in dystonia. Compared to a group of healthy controls, odor identification score as assessed by the University of Pennsylvania Smell Identification Test (UPSIT) in 14 dystonia subjects was slightly lower than in healthy controls, but the difference was not statistically significant (Silveira-Moriyama et al. 2009). In a different study, a statistically lower odor identification score as assessed by the UPSIT was found in five dystonia subjects with mutations in the GNAL gene of only one family (Vemula et al. 2013). More recently, odor identification, odor discrimination and odor threshold were assessed by the Sniffin Sticks (Hummel et al. 1997) in 58 subjects with CD (Marek et al. 2018). Results were compared to data of healthy controls matched only for age and gender from a database. Subjects with CD had lower scores for odor threshold and odor identification, while their score for odor discrimination was in the normal range.
There are several confounding factors that have not or have been only partially taken into account in past studies of olfaction in CD. Sinusoidal disorders can cause olfactory decline (Hummel et al. 2016). Epidemiological factors such as age and gender effect olfaction, but also education and tobacco smoking (Vennemann et al. 2008). Additionally, cognitive domains including executive functioning, memory and verbal fluency impact performance on olfactory tests (Hedner et al. 2010; Westervelt et al. 2005). Psychiatric alterations including depression and anxiety have also been found to impede the sense of smell (Croy and Hummel 2017; Kamath et al. 2018). In terms of olfaction in CD, these factors are possibly important as cognitive and psychiatric alterations are non-motor features of CD (Kuyper et al. 2011).
Little is known about the sense of taste in movement disorder subjects (Cecchini et al. 2015; Lang et al. 2006; Shah et al. 2009). To the best of our knowledge, taste has not been assessed in CD so far. This is surprising as, similar to the sense of smell, the sense of taste contributes to quality of life (Croy et al. 2014; Doty 2019).
To contribute to the better understanding of CD, the primary aim of this study was to systematically examine olfactory and gustatory functioning in CD subjects compared to healthy controls matched for epidemiological factors such as age, sex and education. Diseases which could impede the chemical senses such as sinusoidal disorders were exclusion criteria through history and clinical examination. Also, clinical characteristics of dystonia, cognitive functioning and psychiatric alterations were analyzed as cofactors of olfactory and gustatory performance.
Methods
Study participants
CD subjects were recruited through the Movement Disorder Center of the Department of Neurology at the University Hospital Greifswald. Inclusion criteria for CD subjects were adult age onset idiopathic cervical dystonia consistent with to established criteria (Albanese et al. 2013; ESDE 2000). Excluding criteria were any central nervous system pathologies other than CD, history of or current radiation and chemotherapy, use of central nervous active medication, anatomical deformities or pathologies of mouth, ear and nose or any medical or surgical condition which could impede smell or taste (Heckmann et al. 2003; Landis et al. 2011; Patel and Pinto 2014), a score below 26 in the Montreal Cognitive Assessment (MoCA) (Freitas et al. 2012) as well as history of head trauma and abnormal findings on neuroimaging studies or laboratory work-up as done for routine care. CD subjects treated with botulinum toxin (BTX) were examined at least 3 months after their last BTX treatment. Healthy control subjects were selected to match age, sex, handedness and education of CD subjects. The same exclusion criteria for the selection of CD subjects were applied for the selection of healthy controls. For any CD subject who smoked tobacco, a control subject with a similar smoking burden (number of cigarettes consumed per day) was included.
Clinical interview, examination and scores
Demographic as well as clinical data such as onset of dystonia and family history of a movement disorder were collected during an in-person interview in addition to the review of the clinical charts. The test battery applied in all study participants included the MoCA to assess overall cognitive functioning (Freitas et al. 2012) the Trail-Making-Test (TMT) for the assessment of executive functioning (Brown et al. 1958), the Digit Span Test (DST) (De Paula et al. 2016) to assess short-term memory, the FAS-Test to assess verbal fluency (Machado et al. 2009) and the Brief Symptom Inventory (BSI) (Franke 2000) to assess depression and anxiety. A neurological exam in all study subjects was done by a fellowship-trained, senior movement disorders neurologist (MK). The complete Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) (Consky and Lang 1994) was done in all CD subjects in this study. Tremor was assessed using part one of the Clinical Rating Scale for Tremor (Fahn et al. 1988). When tremor in CD affected the head, the tremor was classified as dystonic tremor (DT), whereas when the tremor affected a different body part, such as the arms, it was classified as tremor associated with dystonia (TAWD) (Bhatia et al. 2018). The nose was inspected by anterior rhinoscopy and the oral cavity was inspected by the use of a tongue depressor and an examination light to rule out other causes of olfactory or gustatory decline (Hummel et al. 2016). All study participants were asked to rate their overall olfactory and gustatory functioning on a scale from zero (absence) to ten (excellent), with five representing an average olfactory and gustatory functioning. The participants were requested not to drink, eat or smoke one hour before the beginning of the olfactory and gustatory examination. All study participants were examined in a well-rested state.
Olfactory testing
The Sniffin Sticks were used to assess olfactory functioning including odor threshold, odor identification and odor discrimination (Hummel et al. 1997). Sniffin Sticks are felt-tip pen-like dispensers that contain odorants. For olfactory testing, the cap was removed and the stick was presented under the nostrils for 3 s. During the entire olfactory assessment, all participants were blindfolded to prevent visual identification.
For odor threshold testing, 16 triplets were used. Each triplet contained one stick with the odorant n-butanol and two sticks with a solvent. The three sticks of a triplet were presented to the study participants in a randomized order. In a three alternative forced choice procedure (3-AFC procedure) they were asked to identify the odor-containing pen. The assessment started with the lowest concentration. If the subjects gave a wrong answer, the triple with the next higher odor concentration was presented. When the stick containing the odor was correctly identified, the same odor concentration was presented again. After two successful trials the next lower concentration was presented. Incorrect answers led to the presentation of the next higher concentration. The reversals between lower to higher or higher to lower concentration were considered inflection points. The odor threshold was defined as the mean concentration of the last 4 of 7 inflection points. To prevent habituation and fatigue, a 30-s break was done between every triplet. Odor discrimination was assessed using 16 triplets with two sticks containing the same odorant and one stick with a different odorant. In a 3-AFC procedure the participants had to choose which pen smelled different to the other two. All triplets were presented in a randomized order and a break of 30 s between every triplet was given. To assess odor identification capability, 16 common odors were presented. The participants could choose one out of four answer options and a 30-3s break was taken between the presentation of the odors. The score range was 0 (poor result) to 16 (excellent result) for each of the three olfactory tests. The sum of the threshold, discrimination, and identification test was added to the composite olfactory score (maximum score 48). A composite olfactory odor score below 30 was defined as hyposmia (Haehner et al. 2009).
Gustatory assessment
The sense of taste was assessed using the Taste Strips (Mueller et al. 2003). These filter paper strips were impregnated with four different taste qualities (sweet, sour, salty and bitter). Every taste was represented in four concentrations, beginning with the lowest. The taste strips were placed on the anterior part of the tongue. The participants were allowed to take a sip of water between every taste strip presented. In total, 18 strips were presented. Among them were two blanks without a taste quality. All strips were presented in increasing concentration and taste qualities were tested in a predetermined pseudo-randomized order. Every time a strip was presented, the participants had to choose an answer from the following options: “sweet”, “sour”, “salty”, “bitter” or “no taste”. The scores of the four taste qualities tested were added to the composite taste score. The results of the blanks were not counted. The score range was 0 (poor result) to 16 (excellent result). A composite taste score below nine was defined as a hypogeusia (Mueller et al. 2003).
Statistics
Statistical analysis was performed using SPSS Statistics 25 software (SPSS Inc., Chicago, IL, USA). The test of Kolmogorov–Smirnov indicated that the data were normally distributed. Independent samples Student t test (two-tailed) was used to compare results of the CD subjects to healthy controls. The chi-square test was applied to compare prevalences. In case of statistically significant differences between CD subjects and healthy controls in the tests for the chemical senses, multiple linear regression analysis in the stepwise mode was done to assess whether clinical characteristics of CD predicted performance of the olfactory or gustatory tests. Factors included in these analyses were age, sex, tobacco smoking, education, disease duration, family history of CD, BTX treatment, TWSTRS part one to three, DT and TAWD as assessed with the CRST part one, performance in the cognitive tests as well as depression and anxiety sub-scores of BSI.
Results
Study participants
Details on the characteristics of the study participants can be found in Table 1. All participants were Caucasian and righthanded. DT was found in 25 of the 40 CD subjects and 12 of these CD subjects with DT had additionally TAWD. Besides the clinical signs and symptoms of CD in the CD subjects, the clinical neurological exam and exam of the nose and mouth were normal in all study participants. There were eight CD subjects with a family member who had CD as well. Thirty CD subjects had obtained BTX in the past, while ten CD subjects never had BTX before.
Olfactory ability
The group of CD subjects had lower scores than the group of healthy controls in the composite olfactory score (Table 2), the odor threshold test and the odor identification test, while no statistically significant difference was found in the odor discrimination test (Table 2). There were more CD subjects than healthy controls with hyposmia (52.5% versus 22.5%, p = 0.003). Subjective olfactory functioning in CD subjects was lower than in healthy controls (5.5 ± 1.9 versus 6.4 ± 1.8, p = 0.04).
Gustatory ability
The combined taste score was lower in CD subjects than in healthy controls (Table 2). There were more CD subjects than healthy controls with hypogeusia (35.0% versus 12.5%, p = 0.03). Subjective gustatory function in CD subjects was not statistically different from healthy controls (5.9 ± 1.8 versus 6.4 ± 1.4, p = 0.1).
Clinical characteristics and chemical senses in CD
Multiple linear regression revealed a significant regression coefficient for age to predict low performance in the composite odor score ((F(1,38) = − 2.4, p = 0.02), R2 = 0.13, standard coefficient beta = − 0.36) and odor identification ((F(1,38) = − 2.7, p = 0.01), R2 = 0.16, standard coefficient beta = − 0.395). Additionally, multiple linear regression showed a significant regression coefficient for lower MoCA score to predict lower performance in the composite taste score in CD subjects ((F(1,38) = − 2.25, p = 0.03), R2 = 0.12, standard coefficient beta = − 0.34) and for TWSTRS—part 3 to predict lower performance in the odor threshold test ((F(1,38) = − 2.4, p = 0.02), R2 = 0.13, standard coefficient beta = − 0.36). All other regression equations did not reach the level of significance (all p values > 0.05).
Discussion
This study systematically investigated the sense of smell and taste in 40 adult subjects with idiopathic CD compared with 40 healthy controls matched for age, sex, education and tobacco use. The main findings are that CD subjects had lower odor threshold, lower odor identification and lower composite taste scores than healthy controls.
Details on the neural circuits involved in odor threshold, odor discrimination and odor identification may help to explain findings. Peripheral olfactory structures such as the olfactory neuroepithelium and nerve are important for odor threshold (Hummel et al. 2016). For instance, diminished odor threshold is typically found in sinusoidal disorders (Patel and Pinto 2014; Whitcroft et al. 2017). While we excluded subjects with sinusoidal disorders, BTX can cause dry mouth (Dalton 2004; Dressler and Benecke 2003) and possibly dry nose, which could impede odor threshold. Although we cannot exclude this possibility, we believe it is less likely. The CD subjects on BTX treatment were assessed 3 months after the last BTX injections when the effects of BTX had worn off. Diminished odor threshold can be found with impairment of an olfacto-motor loop (Mainland et al. 2005). Within this circuit, the cerebellum as a structure for sensorimotor control regulates sniff volume inversely proportional to odor concentration in order to optimize sampling of sensory information (Bower 1997; Mainland et al. 2005; Sobel 1998; Zobel et al. 2010). We do not know if this is the case for CD, as we did not measure nasal air flow during the olfactroy tests. The use of a fixed-sniff method (Mainland et al. 2005) in future studies may reveal whether alterations of this loop contributes to diminished odor threshold in CD.
Functional imaging (Positron Emission Tomography and Magnet Resonance Tomography) in healthy subjects was applied to explore neural circuits involved in higher olfactory functions (Savic et al. 2000; Suzuki et al. 2001; Kareken et al. 2003; Kjelvik et al. 2012). Varying paradigms were used in the different studies, which limits comparability of findings. However, it appears that the olfactory system is organized in both a parallel and hierarchal manner (Savic et al. 2000). For example, the olfactory core regions including the right amygdala-piriform cortex, the right orbito-frontal and the prefrontal cortex, the left insular, the cingulum and the right thalamus were mutually activated by passive smelling of odors (= parallel organization) (Savic et al. 2000; Suzuki et al. 2001; Kareken et al. 2003; Kjelvik et al. 2012). In contrast, with increased complexity of the olfactory task, the activated areas were more and more remotely connected with the olfactory core regions (= hierarchical organization) (Savic et al. 2000). For instance, in contrast to passive smelling of an odor, tasks for odor threshold, odor discrimination and odor identification activated the right cerebellum (Savic et al. 2000). Moreover, some regions were only activated with certain tasks (for example, odor discrimination tasks activated the hippocampus (Savic et al. 2000; Kareken et al. 2003) and the caudate nucleus of the basal ganglia (Savic et al. 2000), while odor identification tasks activated the left cerebellum (Savic et al. 2000; Suzuki et al. 2001; Kjelvik et al. 2012), the right temporal as well as the right parietal cortex (Savic et al. 2000; Suzuki et al. 2001). Clinical studies seem to support these observations. For instance, focal cerebellar lesions caused impairment of odor threshold and odor identification (Mainland et al. 2005; Zobel et al. 2010). In a nutshell, several overlapping neural circuits are involved in the processing of higher olfactory functions. CD may be a network disorder (Prudente et al. 2014; Quartarone and Hallett 2013; Shakkottai et al. 2017) and our findings of olfactory decline in CD support this idea rather than pointing to a single site of pathology in CD.
Standardized gustatory testing revealed diminished perception of taste in CD subjects compared to healthy controls. The reason for this is not completely clear. The participation of the sensorimotor cortex in the pathophysiology of CD (Prudente et al. 2014; Quartarone and Hallett 2013; Shakkottai et al. 2017) and the perception of the tactile components of taste (Mascioli et al. 2015; Wistehube et al. 2018) may play a role in gustatory decline in CD. Olfactory decline can lead to lower gustatory functioning (Landis et al. 2010; Rolls et al. 2005) and may have contributed to the present findings as well.
Similar to findings of studies on the chemical sense in aging, the present data analysis suggests that age may a predictor for lower performance in the olfactory tests in CD (Doty 2018; Hummel et al. 2007; Mueller et al. 2003; Murphy et al. 2002). Also, cognitive functioning may play a role, as lower MoCA scores predicted lower performance with the composite taste score in CD subjects. Interestingly, the TWSTRS—part 3, which assesses the severity of pain in CD, predicted lower performance in the odor threshold test. Confirmation of present findings through additional studies involving more CD subject is warranted.
This study has limitations. Although being the first study on taste in CD and the second largest study on olfaction in CD (Marek et al. 2018) involving a sizable number of subjects to address the primary aim of this study, the number of CD subjects assessed may have been too small to analyze factors with small to moderate impact on the chemical senses. Second, tobacco smokers were allowed into this study. This was done to better reflect the cohort of subjects with CD. However, tobacco smoking (especially ≥ 20 cigarettes per day) negatively impacts the chemical senses (Vennemann et al. 2008). To control for this confounding factor, the same number of healthy subjects with the same smoking burden were included. Our analyses suggest that tobacco smoking may not be the main factor for decline of the chemical senses in CD and confirms findings of an independent study on the sense of smell in CD (Marek et al. 2018). A third limitation is that the assessment of the study participants was done unblinded, which could have caused bias. However, established and validated psychophysical assessment tools were applied by trained examiners for the examination of the chemical senses (Hummel et al. 1997; Mueller et al. 2003). Finally, besides the BSI, which is a widely used, reliable and validated questionnaire (Tarescavage and Ben-Porath 2014), the Structured Clinical Interview for DSM-III-R (SCID) (Spitzer et al. 1992) may be added in future studies on the chemical senses to more precisely quantify psychiatric alteration in CD.
In conclusion, findings propose that CD is associated with diminished olfactory and gustatory functioning. The assessment of a larger number of CD subjects may confirm present findings and to see if an impairment of the chemical senses is a potential endophenotype of CD. Future studies may also assess whether therapeutic intervention such as deep brain stimulation targeting the basal ganglia or BTX impacts the chemical senses in CD.
Change history
06 May 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00702-021-02340-0
References
Albanese A, Bhatia K, Bressman SB, Delong MR, Fahn S, Fung VS, Hallett M, Jankovic J, Jinnah HA, Klein C, Lang AE, Mink JW, Teller JK (2013) Phenomenology and classification of dystonia: a consensus update. Mov Disord 28:863–873
Alexander GE, Delong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381
Bhatia KP, Bain P, Bajaj N, Elble RJ, Hallett M, Louis ED, Raethjen J, Stamelou M, Testa CM, Deuschl G, Tremor Task Force of the International Parkinson, and Movement Disorder Society (2018) Consensus statement on the classification of tremors. from the task force on tremor of the international parkinson and movement disorder society. Mov Disord 33:75–87
Bodranghien F, Bastian A, Casali C, Hallett M, Louis ED, Manto M, Mariën P, Nowak DA, Schmahmann JD, Serrao M, Steiner KM, Strupp M, Tilikete C, Timmann D, van Dun K (2016) Consensus paper: revisiting the symptoms and signs of cerebellar syndrome. Cerebellum 15:369–391
Bostan AC, Dum RP, Strick PL (2013) Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cognit Sci 17:241–254
Bower JM (1997) Control of sensory data acquisition. Int Rev Neurobiol 41:489–513
Brown EC, Casey A, Fisch RI, Neuringer C (1958) Trial making test as a screening device for the detection of brain damage. J Consult Psychol 22:469–474
Cecchini MP, Fasano A, Boschi F, Osculati F, Tinazzi M (2015) Taste in Parkinson’s disease. J Neurol 262:806–813
Connelly T, Farmer JM, Lynch DR, Doty RL (2003) Olfactory dysfunction in degenerative ataxias. J Neurol Neurosurg Psychiatry 74:1435–1437
Consky ES, Lang AE (1994) Clinical assessments of patients with cervical dystonia. In: Jankovic J, Hallett M (eds) Therapy with botulinum toxin. Marcel Dekker, New York, pp 211–237
Conte A, Defazio G, Hallett M, Fabbrini G, Berardelli A (2019) The role of sensory information in the pathophysiology of focal dystonias. Nat Rev Neurol 15:224–233
Croy I, Hummel T (2017) Olfaction as a marker for depression. J Neurol 264:631–638
Croy I, Nordin S, Hummel T (2014) Olfactory disorders and quality of life-an updated review. Chem Senses 39:185–194
Dalton P (2004) Olfaction and anosmia in rhinosinusitis. Curr Allergy Asthma Rep 4:230–236
De Paula JJ, Malloy-Diniz LF, Romano-Silva MA (2016) Reliability of working memory assessment in neurocognitive disorders: a study of the Digit Span and Corsi Block-Tapping tasks. Braz J Psychiatry 38:262–263
Doty RL (2018) Age-related deficits in taste and smell. Otolaryngol Clin N Am 51:815–825
Doty RL (ed) (2019) Epidemiology of smell and taste dysfunction. Handbook of clinical neurology. Elsevier, Amsterdam, pp 3–13
Dressler D, Benecke R (2003) Autonomic side effects of botulinum toxin type B treatment of cervical dystonia and hyperhidrosis. Eur Neurol 49:34–38
Eisinger RS, Urdaneta ME, Foote KD, Okun MS, Gunduz A (2018) Non-motor characterization of the basal ganglia: evidence from human and non-human primate electrophysiology. Front Neurosci 12:385
Epidemiological Study of Dystonia in Europe (ESDE) Collaborative Group (2000) A prevalence study of primary dystonia in eight European countries. J Neurol 247:787–792
Eslinger PJ, Damasio AR, Van Hoesen GW (1982) Olfactory dysfunction in man: anatomical and behavioral aspects. Brain Cogn 1:259–285
Fahn S, Tolosa E, Marin C (1988) Clinical rating scale for tremor. In: Jankovic J, Tolosa E (eds) Parkinson’s disease and movement disorders. Urban and Schwarzenberg, Munich, pp 271–280
Franke GH (2000) BSI: brief symptom inventory—Deutsche version, manual. Beltz, Göttingen
Freitas S, Simões MR, Marôco J, Alves L, Santana I (2012) Construct validity of the Montreal Cognitive Assessment (MoCA). J Int Neuropsychol Soc 18:242–250
Fudge JL, Breitbart MA, Danish M, Pannoni V (2005) Insular and gustatory inputs to the caudal ventral striatum in primates. J Comp Neurol 490:101–118
Gerwig M, Guberina H, Eßer AC, Siebler M, Schoch B, Frings M, Kolb FP, Aurich V, Beck A, Forsting M, Timmann D (2010) Evaluation of multiple-session delay eyeblink conditioning comparing patients with focal cerebellar lesions and cerebellar degeneration. Behav Brain Res 212:143–151
Haehner A, Boesveldt S, Berendse HW, Mackay-Sim A, Fleischmann J, Silburn PA, Johnston AN, Mellick GD, Herting B, Reichmann H, Hummel T (2009) Prevalence of smell loss in Parkinson’s disease—a multicenter study. Parkinsonism Relat Disord 15:490–494
Heckmann JG, Heckmann SM, Lang CJG, Hummel T (2003) Neurological aspects of taste disorders. Arch Neurol 60:667–671
Hedner M, Larsson M, Arnold N, Zucco GM, Hummel T (2010) Cognitive factors in odor detection, odor discrimination, and odor identification tasks. J Clin Exp Neuropsychol 32:1062–1067
Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G (1997) ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 22:39–52
Hummel T, Kobal G, Gudziol H, Mackay-Sim A (2007) Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol 264:237–243
Hummel T, Whitcroft KL, Andrews P, Altundag A, Cinghi C, Costanzo RM, Damm M, Frasnelli J, Gudziol H, Gupta N, Haehner A, Holbrook E, Hong SC, Hornung D, Hüttenbrink KB, Kamel R, Kobayashi M, Konstantinidis I, Landis BN, Leopold DA, Macchi A, Miwa T, Moesges R, Mullol J, Mueller CA, Ottaviano G, Passali GC, Philpott C, Pinto JM, Ramakrishnan VJ, Rombaux P, Roth Y, Schlosser RA, Shu B, Soler G, Stjärne P, Stuck BA, Vodicka J, Welge-Luessen A (2016) Position paper on olfactory dysfunction. Rhinology 58(1):1–30
Ikai Y, Takada M, Shinonaga Y, Mizuno N (1992) Dopaminergic and non-dopaminergic neurons in the ventral tegmental area of the rat project, respectively, to the cerebellar cortex and deep cerebellar nuclei. Neuroscience 51:719–728
Ikai Y, Takada M, Mizuno N (1994) Single neurons in the ventral tegmental area that project to both the cerebral and cerebellar cortical areas by way of axon collaterals. Neuroscience 61:925–934
Junker J, Paulus T, Brandt V, Weissbach A, Tunc S, Loens S, Reilly RB, Hutchinson M, Baumer T (2019) Temporal discrimination threshold and blink reflex recovery cycle in cervical dystonia—two sides of the same coin? Parkinsonism Relat Disord 68:4–7
Kamath V, Paksarian D, Cui L, Moberg PJ, Turetsky BI, Merikangas KR (2018) Olfactory processing in bipolar disorder, major depression, and anxiety. Bipol Disord 20:547–555
Kareken DA, Mosnik DM, Doty RL, Dzemidzic M, Hutchins GD (2003) Functional anatomy of human odor sensation, discrimination, and identification in health and aging. Neuropsychology 17:482–495
Kjelvik G, Evensmoen HR, Brezova V, Håberg AK (2012) The human brain representation of odor identification. J Neurophysiol 108:645–657
Kuyper DJ, Parra V, Aerts S, Okun MS, Kluger BM (2011) Nonmotor manifestations of dystonia: a systematic review. Mov Disord 26:1206–1217
Landis BN, Scheibe M, Weber C, Berger R, Brämerson A, Bende M, Nordin S, Hummel T (2010) Chemosensory interaction: acquired olfactory impairment is associated with decreased taste function. J Neurol 257:1303–1308
Landis BN, Marangon N, Saudan P, Hugentobler M, Giger R, Martin PY, Lacroix JS (2011) Olfactory function improves following hemodialysis. Kidney Int 80:886–893
Lang CJG, Leuschner T, Ulrich K, Stössel C, Heckmann JG, Hummel T (2006) Taste in dementing diseases and Parkinsonism. J Neurol Sci 248:177–184
Machado TH, Fichman HC, Santos EL, Carvalho VA, Fialho PP, Koenig AM, Fernandes CS, Lourenço RA, Paradela EMP, Caramelli P (2009) Normative data for healthy elderly on the phonemic verbal fluency task—FAS. Dement Neuropsychol 3:55–60
Mainland JD, Johnson BN, Khan R, Ivry RB, Sobel N (2005) Olfactory impairments in patients with unilateral cerebellar lesions are selective to inputs from the contralesional nostril. J Neurosci 25:6362–6371
Marek M, Linnepe S, Klein C, Hummel T, Paus S (2018) High prevalence of olfactory dysfunction in cervical dystonia. Parkinsonism Relat Disord 53:33–36
Mascioli G, Berlucchi G, Pierpaoli C, Salvolini U, Barbaresi P, Fabri M, Polonara G (2015) Functional MRI cortical activations from unilateral tactile-taste stimulations of the tongue. Physiol Behav 151:221–229
Mueller C, Kallert S, Renner B, Stiassny K, Temmel AF, Hummel T, Kobal G (2003) Quantitative assessment of gustatory function in a clinical context using impregnated “taste strips”. Rhinology 41(1):2–6
Murphy C, Schubert CR, Cruickshanks KJ et al (2002) Prevalence of olfactory impairment in older adults. J Am Med Assoc 288:2307–2312
Paracka L, Wegner F, Blahak C, Abdallat M, Saryyeva A, Dressler D, Karst M, Krauss JK (2017) Sensory alterations in patients with isolated idiopathic dystonia: an exploratory quantitative sensory testing analysis. Front Neurol 8:553
Patel RM, Pinto JM (2014) Olfaction: anatomy, physiology, and disease. Clin Anat 27:54–60
Prudente CN, Hess EJ, Jinnah HA (2014) Dystonia as a network disorder: what is the role of the cerebellum? Neuroscience 260:23–35
Quartarone A, Hallett M (2013) Emerging concepts in the physiological basis of dystonia. Mov Disord 28:958–967
Rolls ET (2005) Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiol Behav 85:45–56
Russchen FT, Bakst I, Amaral DG, Price JL (1985) The amygdalostriatal projections in the monkey. An anterograde tracing study. Brain Res 329:241–257
Saint-Cyr JA (2003) Frontal-striatal circuit functions: Context, sequence, and consequence. J Int Neuropsychol Soc 9:103–127
Sarasso E, Agosta F, Piramide N, Bianchi F, Butera C, Gatti R, Amadio S, Carro U, Filippi M (2020) Sensory trick phenomenon in cervical dystonia: a functional MRI study. J Neurol. https://doi.org/10.1007/s00415-019-09683-5(Epub ahead of print)
Savic I (2002) Brain imaging studies of the functional organization of human olfaction. Neuroscientist 8:204–211
Savic I, Gulyas B, Larsson M, Roland P (2000) Olfactory functions are mediated by parallel and hierarchical processing. Neuron 26:735–745
Shah M, Deeb J, Fernando M, Noyce A, Visentin E, Findley LJ, Hawkes CH (2009) Abnormality of taste and smell in Parkinson’s disease. Parkinsonism Relat Disord 15:232–237
Shakkottai VG, Batla A, Bhatia K, Dauer WT, Dresel C, Niethammer M, Eidelberg D, Raike RS, Smith Y, Jinnah HA, Hess EJ, Meunier S, Hallett M, Fremont R, Khodakhah K, LeDoux MS, Popa T, Gallea C, Lehericy S, Bostan AC, Strick PL (2017) Current opinions and areas of consensus on the role of the cerebellum in dystonia. Cerebellum 16:577–594
Silveira-Moriyama L, Schwingenschuh P, O'Donnell A, Schneider SA, Mir P, Carrillo F, Terranova C, Petrie A, Grosset DG, Quinn NP, Bhatia KP, Lees AJ (2009) Olfaction in patients with suspected parkinsonism and scans without evidence of dopaminergic deficit (SWEDDs). J Neurol Neurosurg Psychiatry 80:744–748
Sobel N, Prabhakaran V, Hartley CA, Desmond JE, Zhao Z, Glover GH, Gabrieli JD, Sullivan EV (1998) Odorant-induced and sniff-induced activation in the cerebellum of the human. J Neurosci 18:8990–9001
Spitzer RL, Williams JBW, Gibbon M, First MB (1992) The structured clinical interview for DSM-III-R (SCID): I: history, rationale, and description. Arch Gen Psychiatry 49:624–629
Strick PL, Dum RP, Fiez JA (2009) Cerebellum and nonmotor function. Annu Rev Neurosci 32:413–434
Suzuki Y, Critchley HD, Suckling J, Fukuda R, Williams SC, Andrew C, Howard R, Ouldred E, Bryant C, Swift CG, Jackson SH (2001) Functional magnetic resonance imaging of odor identification: the effect of aging. J Gerontol A Biol Sci Med Sci 56:756–760
Tarescavage AM, Ben-Porath YS (2014) Psychotherapeutic outcomes measures: a critical review for practitioners. J Clin Psychol 70:808–830
Tinazzi M, Squintani GM, Bhatia KP, Segatti A, Donato F, Valeriani M, Erro R (2019) Pain in cervical dystonia: evidence of abnormal inhibitory control. Parkinsonism Relat Disord 65:252–255
Vemula SR, Puschmann A, Xiao J, Zhao Y, Rudzińska M, Frei KP, Truong DD, Wszolek ZK, LeDoux MS (2013) Role of Gα(olf) in familial and sporadic adult-onset primary dystonia. Hum Mol Genet 22:2510–2519
Vennemann MM, Hummel T, Berger K (2008) The association between smoking and smell and taste impairment in the general population. J Neurol 255:1121–1126
Weintraub DB, Zaghloul KA (2013) The role of the subthalamic nucleus in cognition. Rev Neurosci 24(2):125–138
Westervelt HJ, Ruffolo JS, Tremont G (2005) Assessing olfaction in the neuropsychological exam: the relationship between odor identification and cognition in older adults. Arch Clin Neuropsychol 20:761–769
Whitcroft KL, Cuevas M, Haehner A, Hummel T (2017) Patterns of olfactory impairment reflect underlying disease etiology. Laryngoscope 127:291–295
Wistehube T, Rullmann M, Wiacek C, Braun P, Pleger B (2018) Fat perception in the human frontal operculum, insular and somatosensory cortex. Sci Rep 8:11825–11833
Zobel S, Hummel T, Ilgner J, Finkelmeyer A, Habel U, Timmann D, Schulz JB, Kronenbuerger M (2010) Involvement of the human ventrolateral thalamus in olfaction. J Neurol 257:2037–2043
Acknowledgements
We thank Professor Ulf Schminke, Department of Neurology, University of Greifswald, Greifswald, Germany for critical comments on the manuscript. Additionally, we would like to thank Rosalie L. Donaldson-Kronenbuerger for English language editing. The study was funded by internal grants of the Department of Neurology, University of Greifswald, Greifswald, Germany.
Author information
Authors and Affiliations
Contributions
Conceptualization: TH, MK, TH, BL; methodology: TH, MK, TH, BL; formal analysis and investigation: TH, MK, TH, MV; writing—original draft preparation: TH; writing—review and editing: TH, TH, MV, CW, BV, JG, RF, BL, MJ-U, AS, MK; funding acquisition: MK; resources: TH, MK; supervision: MK.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All procedures performed in this study were in accordance with the ethical standards of the University of Greifswald, Faculty of Medicine (study reference number: BB 188/17) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All study participants gave their informed consent prior to their inclusion in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to a retrospective Open Access order.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Herr, T., Hummel, T., Vollmer, M. et al. Smell and taste in cervical dystonia. J Neural Transm 127, 347–354 (2020). https://doi.org/10.1007/s00702-020-02156-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-020-02156-4