Myasthenia gravis (MG) is an autoimmune disease characterized by muscle weakness and fatigue, which is caused by autoantibodies directed against acetylcholine receptors (AChR) at the neuromuscular junction [1]. The prognosis is relatively favorable with optimum symptomatic, immunosuppressive and supportive treatment. Pyridostigmine is the preferred symptomatic treatment, and corticosteroids, azathioprine and thymectomy are first-line immunosuppressive therapies [2].
Guillain-Barré syndrome (GBS) is the most common and most severe acute paralytic neuropathy, with about 100,000 people developing the disorder every year worldwide [3]. Under the terms of GBS are several recognizable variants with distinct clinical and pathological features. As a rare variant of GBS, Miller Fisher syndrome (MFS) is an immune-mediated neuropathy that involves the triad of symptoms of acute ophthalmoplegia, ataxia and areflexia, also with positive GQ1b antibody. The current available treatments include intravenous immunoglobulin (IVIG), plasmapheresis, and supportive care including treatment of underlying infections and physical therapy [4]. MFS usually runs a benign clinical course, with case fatality of < 5% [5].
Up to now, the occurrence of MG and GBS overlapping in the same patient is quite scarce. To our best of knowledge, only four cases have previously been reported regarding the temporal coincidence between MG and MFS [6–9]. Here, we review all the above 4 cases, and we also describe a new case of our own. We also aimed to summarize the clinical characteristics and to elucidate the underlying mechanisms in such a rare overlapping syndrome.
Literature was reviewed through the databases of PubMed, Embase, Cochrane Library and Science Direct from January 1982 to June 2017, and the articles were restricted to those published in English. Key search terms included “Guillain-Barré syndrome”, “Miller Fisher syndrome” and “myasthenia gravis”. Patients with combined MG and MFS were identified and their clinical data such as gender, age, nationality, past history, precipitating factors, clinical presentations, laboratory examinations, cerebrospinal fluid (CSF) findings, AChR antibody, anti-GQ1b antibody, thymoma, treatment and prognosis during follow-up were all investigated in detail.
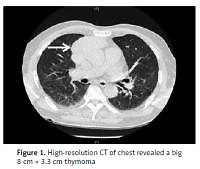
We herein present a case with a temporal coincidence between MG and MFS. A 72-year-old man first presented with acute bilateral ptosis, ophthalmoplegia, diplopia and dysphagia for one week. He was healthy except with a smoking history of 30 years. No precipitating upper respiratory or gastrointestinal infective symptoms were found. Neurological examination revealed bilateral ptosis, ophthalmoplegia, bulbar palsy, slight weakness of limbs, areflexia, limb ataxia, and no response of plantar flexor reflexes. Magnetic resonance imaging (MRI) of the patient’s brain and spinal cord yielded normal findings. The serum biomarkers of tumor and paraneoplastic syndrome were normal. The CSF showed an intracranial pressure of 90 mm H20 (reference range: 80–180), with protein 88 mg/dl (reference range: 20–40) and leukocytes 4 cells/mm3 (reference range: 0–8). High-resolution computed tomography (CT) of the chest revealed a huge 8 cm × 3.3 cm thymoma (Figure 1). The neurophysiological tests were performed after 10 days of his admission. Nerve conduction test (NCT) showed mild extended latency of bilateral median nerve, and conduction velocity reduction in the bilateral median nerve; however, the nerve conduction results (tibial and sural nerve) of the lower extremities were within normal limits. The repetitive nerve stimulation (RNS) test indicated decremental responses both at 2–5 Hz and 10–30 Hz stimulation. The prostigmine test was positive, with the partial improvement of the bilateral ptosis and external ophthalmoplegia. Positive anti-AChR and anti-GQ1b antibody were observed from blood serum; however, the other antibodies were negative. The patient underwent oral pyridostigmine (60 mg three times one day) and IVIG treatment for 5 days. However, these two strategies did not improve the patient’s symptoms prominently. About 3 months later, this patient underwent thymectomy at the department of thoracic surgery in our hospital. Thymoma was also confirmed by histopathological examination. After the thymectomy, he was also treated with pyridostigmine (60 mg three times in 1 day) and he recovered fully with a good prognosis in the subsequent 6 months of follow-up.
Of 5 cases, 2 cases had preceding factors. Elevated CSF protein level without pleocytosis (albumino-cytologic dissociation) was found in 3 cases, there were 3 cases with reduced NCT, 4 cases with positive RNS, 4 cases with positive anti-AChR antibodies, 5 cases with positive anti-GQ1b antibody, and 1 case with coexisting with thymoma. The treatment included pyridostigmine in 3 cases, prednisolone in 1 case, intravenous immunoglobulin in 3 cases, and plasmapheresis in 1 case. The functional outcome was favorable according to the adopted scale by Hughes (0–1) (Table I).
Table I
Demographic and clinical characteristics of the overlapping MFS and MG
[i] MG – myasthenia gravis, MFS – Miller Fisher syndrome, IVIG – intravenous immunoglobulin, NCT – nerve conduction test, RNS – repetitive nerve stimulation. Functional outcome was ranked according to the adopted scale by Hughes: 0, healthy; 1, minor symptoms or signs, able to run; 2, able to walk > 5 m without assistance, but unable to run; 3, able to walk > 5 m with assistance; 4, bed- or chair-bound; 5, requiring assisted ventilation for at least part of the day, and 6, dead. “+” the patient had preceding factors from infectious diseases while “–” the patient had no preceding factors from infectious diseases. As for the other parameters in Table I, “+” positive findings while “–” negative findings.
As for our case, our patient presented with acute bilateral ptosis, diplopia, ophthalmoplegia, ataxic gait, bulbar dysfunction, weakness of limbs and areflexia. MG was diagnosed according to the clinical features, electrophysiological data, positive anti-AChR antibody and radiological findings of thymoma. The diagnosis of MFS was established based on the classic triad of ataxia, areflexia, and ophthalmoplegia, albumino-cytologic dissociation in CSF, and also confirmed by positive anti-GQ1b antibody. As a result, according to the clinical characteristics, electrophysiological findings, laboratory data, the improvement of anti-acetylcholinesterase and IVIG, especially the effect of thymectomy, the diagnosis of the coincidence between MG and MFS was therefore established.
MG and MFS are well-described autoimmune disorders, which are accepted to be heterogeneous with autoantibodies directed against the neuromuscular junction and peripheral nerve with several different antigens, respectively. However, the exact causes of MG or MFS have remained unclear. The annual incidence of MG ranges from 3 to 30 per 1,000,000 [10]. The annual incidence of MFS is around one patient per one million population [5]. Furthermore, the combination of MG and MFS is extremely rare. Although MG and MFS may have some clinically similar symptoms, the differential diagnosis should be made based on the typical clinical characteristics, and different neurophysiological findings. The serological confirmation with the anti-GQ1b antibody is available and allows for greater diagnostic certainty in the face of confounding symptoms.
Autoimmunity response may play a vital role in understanding the pathophysiological mechanism in both MG and MFS. MFS has been reported to arise through molecular mimicry with microbial oligosaccharides [11]. The theory of molecular mimicry postulates that infectious agents and self-antigen initiate the process of MG and MFS concurrently. It has also been speculated that pathogenic activation of the immune system may lead to the production of cross-reacting antibodies against peripheral nerve and AChR at neuromuscular junctions from the evidence of the experimental findings [12]. Additionally, anti-GQ1b antibodies lead to impaired neuromuscular transmission in a mouse diaphragm model [13]. Another in-vitro study using mouse hemidiaphragm also demonstrated that the neuromuscular junction is an antigenic target and primary site of pathology underlying in MFS [11]. Furthermore, some clinical evidence also supports the presence of molecular mimicry between gangliosides and antecedent infectious agents in patients with GBS.
Another hypothesis proposed is that thymoma or thymus hyperplasia-associated multiorgan autoimmunity may also play an important role in the process of autoimmunity. It has been reported that approximately 8–15% of MG are complicated by autoimmune diseases such as immune thyroid disease and systemic lupus erythematosus, which has been well described [14]. The association of MG or MFS with some other autoimmune diseases such as autoimmune thyroiditis has also been previously described [15–17]. In our study, only one patient suffered from thymoma; however, the mass of thymoma. may be considered to be a commonly involved immune organ of MG.
In conclusion, we herein described a case involving the overlap of MG and MFS. To our best of knowledge, the comorbidity of MG and MFS is even rarer with only four reported cases. Some hypotheses are discussed; however, the underlying mechanisms remain to be elucidated in the future.