Abstract
Objectives
To assess the safety and efficacy of percutaneous microwave ablation (MWA) of histologically proven T1 renal cell carcinoma (RCC).
Methods
We analysed patients with a histologically proven RCC (≤ 7 cm) treated by MWA from April 2012–April 2018. Primary and secondary efficacy, local tumour recurrence (LTR), morbidity and mortality were reported. Efficacy was defined as no residual tumour enhancement on follow-up imaging 1 month after the first ablation (primary efficacy) and after re-ablation(s) for residual disease (secondary efficacy). Adverse events (AE) were registered by the Clavien–Dindo classification and the common terminology criteria for AE. Univariable and multivariable logistic regression analyses were performed to investigate a relation among pre-treatment factors incomplete ablation and complications.
Results
In 100 patients, a total of 108 RCCs (85 T1a and 23 T1b) were treated by MWA. Median size was 3.2 cm (IQR 2.4–4.0). Primary efficacy was 89% (95%CI 0.81–0.94) for T1a lesions and 52% (95%CI 0.31–0.73) for T1b lesions (p < 0.001). Fifteen lesions (7 T1a) were re-ablated for residual disease by MWA in one (n = 13) and two (n = 2, both T1b) sessions resulting in secondary efficacy rates of 99% (T1a) and 95% (T1b, p = 0.352). LTR occurred in four tumours (2 T1a, 2 T1b) after 10–60 months. Six (4%) AEs grade > 3–5 were observed (2 T1a, 4 T1b, p = 0.045). Multivariable analysis showed that mR.E.N.A.L. nephrometry was independently associated with incomplete ablation (p = 0.012).
Conclusion
Microwave ablation is safe and effective for T1a and T1b RCC lesions with a significantly lower primary efficacy for T1b lesions.
Similar content being viewed by others
Introduction
Renal cell carcinoma (RCC) accounts for 3% of all cancers worldwide [30]. According to the European Association of Urology (EAU) and the National Comprehensive Cancer Network (NCCN) RCC guidelines, partial nephrectomy (PN) is the gold standard for T1a RCC. Percutaneous tumour ablation is reserved for co-morbid patients and patients not eligible for surgery [21, 25]. Although reports show higher local control after PN compared to ablative therapies, similar cancer-specific survival is obtained with less renal function decline for radiofrequency ablation (RFA) and cryoablation (CA) [32].
Details of the different ablation modalities, RFA, MWA and CA, are extensively described in the literature. To summarize, MWA, compared to RFA, achieves higher temperatures in a shorter time less influenced by the heat sink effect. As a result, a fast and large ablation zone with a similar applicator as RFA is achieved. [4, 20] With RFA and MWA, the evolvement of the ablation zone during the procedure is less visible compared to CA [16]. Reports about the efficacy and safety of large cohorts of MWA remain limited, especially for T1b tumours [9, 10].
In this retrospective cohort study, we report the outcomes of patients with a histologically proven RCC treated by means of MWA in a tertiary reference centre. The purpose of this study was to evaluate the safety, efficacy and factors influencing outcome of MWA in T1a and T1b RCC.
Patients and Methods
Study Population
The institutional review board of our hospital approved this retrospective study (IRBd18059). The data of all MWAs of our institute were requested through our institutes data desk, and consent of all patients was checked. We included patients treated by MWA for a histologically proven T1 RCC between April 2012 and April 2018. Patients were excluded when prior therapy (chemotherapy, surgical resection or a different ablation modality) for RCC was administrated.
MWA Procedure
All patients were first discussed in a multidisciplinary tumour board, consisting of urologists, radiologists and medical oncologists to decide patients eligibility for MWA. The MW procedures were performed computed tomography (CT) guided (CT Somatom Sensation Open, Siemens®, Munchen, Germany). Patients were treated with two different MW systems (2012–2014: Evident® MW system (Covidien®, Dublin, Ireland), 2014–2018: Emprint® MW system (Medtronic®, Dublin, Ireland)). Dissection was performed with 5% glucose solution plus 10% iodinated contrast, CO2 and room air for tumours adjacent to vulnerable structures. Ureteric perfusion with cooled saline was used for tumours close to the collection system and the proximal ureter. Antenna placement was performed with CT fluoroscopy, and optimal position was verified by CT before start of the ablation. After antenna placement, biopsy was performed. In principle, a power of 100 W was used for 2–10 min according to tumour size. A margin of 5–10 mm was attempted to achieve complete ablation. Fluoroscopic CT check was performed to monitor the procedure. In larger tumours, the ablation was repeated with different antenna positions to achieve a complete ablation zone. With the Evident® MW system, multiple antennas were placed in the tumour according to physicians choice.
Follow-Up
An institutional follow-up scheme of multiphase CT scans after 1, 3, 6, 9 and 12 months was executed. Patients with a diminished renal function were followed by (non-)contrast enhanced magnetic resonance imaging (Achieva or Ingenia, Philips Healthcare®, Best, the Netherlands). According to agreement with our in-house urologists, follow-up for the first year was performed by the IR at our outpatient clinics. When no recurrence appeared, patients were sent back to the referring urologist. A follow-up scheme of 1 multiphase CT scan a year for 5 years was advised to the referring urologist.
Data Collection and Statistical Analysis
Tumour characteristics were scored according to the R.E.N.A.L. nephrometry score (radius, exophytic/endophytic, nearness to collecting system or sinus and location relative to polar lines) and modified (m)R.E.N.A.L. nephrometry score, as published previously [26]. Adverse events (AEs) during ablation were registered by the common terminology criteria for adverse events (CTCAE), and post-ablation AEs were registered following the Clavien–Dindo classification. Primary efficacy was defined as no residual tumour enhancement visible at post-contrast CT or MRI 1 month post-ablation. Secondary efficacy was described as the percentage of tumours successfully treated for residual disease by repeated MWA(s) [1]. Patients treated with another secondary treatment modality (i.e. PN, radical nephrectomy (RN), RFA or CA) were excluded for the secondary efficacy. Local tumour recurrence (LTR) was defined when new enhancement within a successfully treated ablation zone occurred during the follow-up.
Continuous variables are shown as median and interquartile ranges (IQR) and categorical data as numbers and percentages. To test differences between categories, the Chi-square or Fisher exact test was used and for nonparametric continuous variables the Mann–Witney test. To analyse the relationship between pre-treatment factors with incomplete ablations and the occurrence of complications, a logistic regression analysis was performed. Results are presented as odds ratio (OR), 95% confidence interval (CI) and significance levels. For the multivariable logistic regression analysis, only significant variables from the univariable analysis were included. Significance levels of p < 0.05 were used. Analyses were performed using Statistical Package for the Social Sciences (SPSS, version 25, Chicago, IL).
Results
Patient and Tumour Characteristics
Between April 2012—April 2018, 226 patients underwent a MWA for their renal masses. One hundred and twenty-six patients were excluded because of non-diagnostic biopsy (n = 55), benign biopsy (n = 30), treatment for recurrence disease after prior treatment (n = 12), metastatic disease (n = 23), T3 disease (n = 2), tumour debulking (n = 2), prior chemotherapy for tumour reduction (n = 1) and no follow-up imaging available after the MWA (n = 1). One hundred patients with 108 histologically proven RCCs were included in this analysis; patient and tumour characteristics are shown in Table 1.
Primary and Secondary Efficacy
A total of 125 MW ablations were performed in 108 tumours. The Evident® MW system was used in 14 MWAs with the use of multiple antennas (2–3) in 10 tumours. The Emprint® MW system was used in the other 111 MWAs. Dissection was used in 37% of the procedures with five ureter perfusions. Patients were mostly placed in the CT scanner in a prone position (75%), under epidural (98%) or general (2%) anaesthesia.
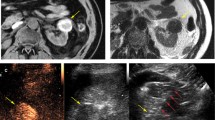
Primary efficacy was achieved in 88 lesions (81%) (Table 2, Fig. 1A–C). T1a lesions had a significantly higher primary efficacy (89%; CI 0.81–0.94) compared to T1b lesions (52%; CI 0.31–0.73) (p < 0.001). Fifteen tumours (53% T1b) received a second MWA and two T1b tumours a third MWA. Secondary efficacy of MWA was reached in 97% (101/103) (all tumours), 99% (82/83) (T1a) and 95% (19/20) (T1b, p = 0.352). Five tumours (5/20, 2 T1a and 3T1b) were not re-treated by MWA and excluded for the secondary efficacy, but were all successfully treated by means of surgery (PN; T1a) and other ablative techniques (RFA (1 T1a tumour (1 × re-RFA) and 1 T1b tumour (3x re-RFA)), CA (n = 1; T1b)) and no treatment (patients choice). In five lesions, the second MWA was incomplete (1 T1a, 4 T1b) and were successfully treated with MWA (n = 2). In three lesions, another ablative modality was used (CA (n = 2) and RFA (n = 1)).
A–C Microwave ablation (MWA) of a T1b tumour (A) before MWA (B) during MWA (C) 1 year after MWA: complete ablation. D–F endophytic T1a lesion with a close relation to the collecting system (D). E + F 9 months after complete ablation, hydronephrosis of the kidney visible due to an urinary tract stenosis that occurred 3 months after the MWA (kidney function from 45 to 19 ml/min/1.73m2) (NB this patient is familiar with liver cysts)
Adverse Events
A total of 24 (19%) AEs were observed after the 125 MW procedures including six major AEs (4%) with a significantly higher number of major AEs in T1b tumours (2 T1a 2% and 4T1b 13%, p = 0.045) (Table 3). There was no significant difference in the occurrence of all complications and T stage (18 T1a 20% and 6 T1b 18%, p = 1.000). One major AE consisted of an active bleeding on the control scan during the MWA, which was successfully coiled embolized (CTCAE grade 3). One patient died 13 days after the ablation due to cardiac and renal failure (Clavien–Dindo grade 5). In two patients, a urinary tract stenosis arose after the MWA (Clavien–Dindo 4a) after 1 and 2 months resulting in a non-functional kidney (see Fig. 1D–F). In three patients, a urinary tract stenosis occurred 1, 2 and 5 months after the MWA without loss of renal function (Clavien–Dindo 1). All five lesions were endophytic T1a lesions, with a close relation to the collection system (4 lesions < 4 mm, 1 lesions 4–7 mm). Cooling of the urinary tract system was performed in one lesion. One patient with macroscopic haematuria required transfusion with packed red blood cells and antibiotics (both grade 3). Nineteen (15%) minor AEs occurred (Clavien–Dindo grade 1 + 2).
Factors Influencing Outcome
R.E.N.A.L. nephrometry score (OR 1.56, p = 0.001), mR.E.N.A.L. nephrometry score (OR 1.58, p = 0.000) and tumour aetiology (clear cell vs non-clear cell OR 0.11, p = 0.034) were significantly associated with an incomplete ablation; however, only the mR.E.N.A.L. nephrometry score remained significantly associated in the multivariable regression analysis (OR = 3.854 p = 0.012) (Table 4). Of the mR.E.N.A.L nephrometry score, size (> 3 and > 4), nearness to the collecting system (< 4 mm) and distance to the polar lines (score 3) were significantly associated with an incomplete ablation.
Univariable analysis showed that only the R.E.N.A.L. nephrometry score (OR 1.308, p = 0.013) and mR.E.N.AL. nephrometry score (OR 1.577, p = 0.016) were associated with the occurrence of complications. Of the (m)RENAL nephrometry score, only the nearness to the collecting system (< 4 mm) was significantly associated with the occurrence of complications.
Follow-Up
Median follow-up time was 19 months (IQR 12–35 months; min–max 0–78 months, 90 patients (90%) 1-year follow-up available). During this period, four (4%) tumours showed LTR (2 T1a and 2 T1b tumours) after 10, 13, 26 and 60 months. One recurrence was successfully treated by MWA and two recurrences by another modality (CA). One of the recurrences has not been treated yet. One patient treated by MWA for bilateral chromophobe RCC tumours developed a new lesion in the same kidney for which he underwent active surveillance. Five patients (4 T1a tumours, 1 T1b tumours) developed metastases of which two were histologically proven RCC. During follow-up, no patient died of RCC.
Discussion
Percutaneous ablation is considered as treatment option in co-morbid patients with a T1 RCC tumour not eligible for PN [21, 25]. RFA and CA are widely applied and established ablation techniques for RCC that are included in the guidelines contrary to MWA which has still limited supportive data [21, 25, 27].
In this study, we show the result of 108 RCCs treated with MWA. There was a significantly higher primary efficacy for T1a tumours (89%) compared to T1b tumours (52%). In the 125 performed MWAs, 19% AEs were observed, mostly low grade (15%), with a significantly higher number of major AEs in T1b tumours (13% T1b vs 2% T1a, p = 0.045). The R.E.N.A.L. score and mR.E.N.A.L. nephrometry score were related to incomplete first ablation and the occurrence of complications. The factors, size (> 3 cm and > 4 cm), nearness to the collecting system (< 4 mm) and distance to the polar lines (score 3) of the mR.E.N.A.L score were associated with incomplete ablation and nearness to the collecting system (< 4 mm) for the occurrence of complications.
For all primary MWAs, a primary efficacy of 81% was observed with a primary efficacy of 89% for T1a tumours. After repeated MWA(s), a secondary efficacy of 99% was reached for T1a tumours. In the literature, primary efficacy rates of MWA from 84.6 to 100% are reported [8, 11, 14, 22, 29]. Therefore, the primary and secondary efficacy of T1a tumours underpins the existing evidence supporting MWA for the treatment of T1a RCC lesions.
The primary efficacy of T1b tumours (52%) was significantly lower compared to T1a tumours (p < 0.001). In 48% of the T1b lesions, a second or third ablation was performed to achieve a complete tumour ablation resulting in a secondary efficacy of 95%. In the literature, lower efficacy rates of percutaneous ablation are described for tumours over 4 cm [29] which is in line with our findings. We show that repeated MW ablations can achieve high efficacy rates even in large tumours. Besides the tumour size, our cohort contained difficult tumours with a close relation to the collecting system. Reports of percutaneous ablation of T1b RCCs by MWA are rare, and series are small [3, 9]. Primary efficacy rates between 75 and 100% were reported in 12 and 7 T1b tumours, respectively [12, 33]. In the literature, CA is more commonly used as ablation technique for T1b lesions with primary efficacy rates ranging from 76 to 97.2% [2, 5, 13, 15]. Our results show that MWA can also be used and chosen as a treatment modality in T1b tumours with consideration of a second ablation. Future cost-effectiveness studies have to show the exact place of the difference ablation techniques for RCC.
The overall AE rate of 19% was relatively high compared to previous percutaneous ablation and surgical studies [9, 32]. However, most AEs were low grade (15%) with minimal consequences for the patient. In our series, we found a significant difference between T1a and T1b tumour for the occurrence of major AEs. Five patients had a stenosis of the urinary tract, 1–5 months after MWA that resulted in renal function loss in two patients. Nearness to the collecting system was significantly associated with incomplete ablation and complications. Therefore, we suggest caution during MWA for lesions close to the collecting system. In the literature, 13 cases of injury to the urinary tract system after MWA are reported [7, 11, 19, 23, 31, 33]. Klapperich et al. observed six asymptomatic urinomas that resulted in renal cortex volume loss in three patients [19]. Preclinical work on a histologically level shows damage of MWA to the collecting system by direct puncture of the collecting system and heating of the urine during the ablation [24, 28]. Damage to the gastrointestinal tract was reported by others, but not observed in the current study [16, 18].
Multivariable analysis showed an independent association of the mR.E.N.A.L. nephrometry score for an incomplete first ablation. In addition, univariable analysis showed a relation of R.E.N.A.L. and m.R.E.N.A.L. nephrometry score with the occurrence of complications. These results are in line with Ierardi et al. that also reported an association of the (m)R.E.N.A.L. nephrometry score with incomplete ablation and complications after MWA [17] and Camacho et al. that first described this association after RFA and CA [6]. On the contrary, Klapperich et al. only found an association between local recurrence after MWA and tumour histology characteristic, and not between the R.E.N.A.L. score and local recurrence [19]. Shakeri et al. observed a significantly higher median tumour size in lesions that required a second MWA, but no association with tumour location and R.E.N.A.L score [29]. Also, Wells et al. did not find an association with the RENAL score and treatment outcome [33]. These reports are all opposite to our findings which may suggest that MWA is not as straight forward as previously reported.
The LTR in this study was 4% over a 108 histologically proven RCCs. In the literature, LTR ranges from 0 to 17% [7, 12, 29], but most papers included every renal lesions without excluding non-diagnostic or benign lesions whereby efficacy and recurrence rates might be overestimated [23, 31, 33]. Surprisingly, the time to LTR was long in this current cohort which could be explained by the difficulties in detecting of recurrences in hypo-vascular tumours and slow growth rates of low-grade tumours.
Limitations of this study include a single-centre retrospective study whereby details of the MW procedure were not complete with the lack of in-house long-term follow-up (> 1 year) in some patients. Ideally, we would describe a larger cohort, but the peri-operative biopsy strategy resulted in some non-diagnostic and benign lesions.
In conclusion, primary efficacy of MWA in T1a tumours was significantly higher compared to T1b tumours. Repeated ablation was necessary in 19% achieving efficacy rates of 95% (T1a) and 99% (T1b). Low-grade AEs were seen after MWA whereby close monitoring of the urinary tract is recommended following ablation of tumours adjacent to the urinary tract. Incomplete ablation was more often seen in lesions with a larger size with a close relation to the collecting system and the polar lines accompanied expressed in a higher mR.E.N.A.L. nephrometry score. Prospective data have to determine the exact position of MWA for the treatment of RCC.
Abbreviations
- RCC:
-
Renal cell carcinoma
- EAU:
-
European association of urology
- NCCN:
-
National comprehensive cancer network
- PN:
-
Partial nephrectomy
- RFA:
-
Radiofrequency ablation
- CA:
-
Cryoablation
- MWA:
-
Microwave ablation
- R.E.N.A.L.:
-
Radius, exophytic/endophytic, nearness to collecting system or sinus and location relative to polar lines
- mR.E.N.A.L.:
-
Modified R.E.N.A.L
- AE:
-
Adverse event
- CTCAE:
-
Common terminology criteria for adverse events
- LTR:
-
Local tumour recurrence
- CT:
-
Computed tomography
- HR:
-
Hazard ratio
- IQR:
-
Interquartile range
- RN:
-
Radical nephrectomy
- ROC:
-
Receiver operating characteristics
- AUCs:
-
Area under the curve
References
Ahmed M. Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update. Radiology. 2014;273(1):241–60.
Atwell TD, Vlaminck JJ, Boorjian SA, et al. Percutaneous cryoablation of stage T1b renal cell carcinoma: technique considerations, safety, and local tumor control. J Vasc Interv Radiol. 2015;26(6):792–9.
Best SL, Park SK, Youssef RF, et al. Long-term outcomes of renal tumor radio frequency ablation stratified by tumor diameter: size matters. J Urol. 2012;187(4):1183–9.
Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol. 2009;38(3):135–43.
Buy X, Lang H, Garnon J, Sauleau E, Roy C, Gangi A. Percutaneous renal cryoablation: prospective experience treating 120 consecutive tumors. AJR Am J Roentgenol. 2013;201(6):1353–61.
Camacho JC, Kokabi N, Xing M, et al. R.E.N.A.L. (Radius, exophytic/endophytic, nearness to collecting system or sinus, anterior/posterior, and location relative to polar lines) nephrometry score predicts early tumor recurrence and complications after percutaneous ablative therapies for renal cell carcinoma: a 5-year experience. J Vasc Interv Radiol. 2015;26(5):686–93.
Castle SM, Salas N, Leveillee RJ. Initial experience using microwave ablation therapy for renal tumor treatment: 18-month follow-up. Urology. 2011;77(4):792–7.
Chan P, Velasco S, Vesselle G, et al. Percutaneous microwave ablation of renal cancers under CT guidance: safety and efficacy with a 2-year follow-up. Clin Radiol. 2017;72(9):786–92.
Choi SH, Kim JW, Kim JH, Kim KW. Efficacy and safety of microwave ablation for malignant renal tumors: an updated systematic review and meta-analysis of the literature since 2012. Korean J Radiol. 2018;19(5):938–49.
Cornelis FH, Marcelin C, Bernhard JC. Microwave ablation of renal tumors: a narrative review of technical considerations and clinical results. Diagn Interv Imaging. 2017;98(4):287–97.
Dong X, Li X, Yu J, Yu MA, Yu X, Liang P. Complications of ultrasound-guided percutaneous microwave ablation of renal cell carcinoma. Onco Targets Ther. 2016;9:5903–9.
Gao Y, Liang P, Yu X, et al. Microwave treatment of renal cell carcinoma adjacent to renal sinus. Eur J Radiol. 2016;85(11):2083–9.
Gunn AJ, Joe WB, Salei A, et al. Percutaneous cryoablation of stage T1b renal cell carcinoma: safety, technical results, and clinical outcomes. Cardiovasc Interv Radiol. 2019;42(7):970–8.
Hao G, Hao Y, Cheng Z, et al. Local tumor progression after ultrasound-guided percutaneous microwave ablation of stage T1a renal cell carcinoma: risk factors analysis of 171 tumors. Int J Hyperth. 2018;35(1):62–70.
Hebbadj S, Cazzato RL, Garnon J, et al. Safety considerations and local tumor control following percutaneous image-guided cryoablation of T1b renal tumors. Cardiovasc Interv Radiol. 2018;41(3):449–58.
Hinshaw JL, Lubner MG, Ziemlewicz TJ, Lee FT Jr, Brace CL. Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation–what should you use and why? Radiographics. 2014;34(5):1344–62.
Ierardi AM, Puliti A, Angileri SA, et al. Microwave ablation of malignant renal tumours: intermediate-term results and usefulness of RENAL and mRENAL scores for predicting outcomes and complications. Med Oncol. 2017;34(5):97.
Janzen NK, Perry KT, Han KR, et al. The effects of intentional cryoablation and radio frequency ablation of renal tissue involving the collecting system in a porcine model. J Urol. 2005;173(4):1368–74.
Klapperich ME, Abel EJ, Ziemlewicz TJ, et al. Effect of tumor complexity and technique on efficacy and complications after percutaneous microwave ablation of stage T1a renal cell carcinoma: a single-center, retrospective study. Radiology. 2017;284(1):272–80.
Laeseke PF, Lee FT Jr, Sampson LA, van der Weide DW, Brace CL. Microwave ablation versus radiofrequency ablation in the kidney: high-power triaxial antennas create larger ablation zones than similarly sized internally cooled electrodes. J Vasc Interv Radiol. 2009;20(9):1224–9.
Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European association of urology guidelines on renal cell carcinoma: the 2019 update. Eur Urol. 2019;75(5):799–810.
Maciolek KA, Abel EJ, Posielski NM, et al. Tumor location does not impact oncologic outcomes for percutaneous microwave ablation of clinical T1a renal cell carcinoma. Eur Radiol. 2019;29(11):6319–29.
Mansilla AV, Bivins EE Jr, Contreras F, Hernandez MA, Kohler N, Pepe JW. CT-guided microwave ablation of 45 renal tumors: analysis of procedure complexity utilizing a percutaneous renal ablation complexity scoring system. J Vasc Interv Radiol. 2017;28(2):222–9.
Moore C, Salas N, Zaias J, Shields J, Bird V, Leveillee R. Effects of microwave ablation of the kidney. J Endourol. 2010;24(3):439–44.
Motzer RJ, Jonasch E, Agarwal N, et al. Kidney cancer, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2017;15(6):804–34.
Mouli SK, McDevitt JL, Su YK, et al. Analysis of the RENAL and mRENAL scores and the relative importance of their components in the prediction of complications and local progression after percutaneous renal cryoablation. J Vasc Interv Radiol. 2017;28(6):860–7.
Prins FM, Kerkmeijer LGW, Pronk AA, et al. Renal cell carcinoma: alternative nephron-sparing treatment options for small renal masses, a systematic review. J Endourol. 2017;31(10):963–75.
Schmitz JJ, Schmit GD, Viers BR, Atwell TD. Renal microwave ablation resulting in ureteropelvic junction stricture remote from the ablation site. J Vasc Interv Radiol. 2017;28(9):1278–80.
Shakeri S, Afshari Mirak S, Mohammadian Bajgiran A, et al. The effect of tumor size and location on efficacy and safety of US- and CT- guided percutaneous microwave ablation in renal cell carcinomas. Abdom Radiol (NY). 2019;44(6):2308–15.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30.
Thompson SM, Schmitz JJ, Thompson RH, et al. Introduction of microwave ablation into a renal ablation practice: valuable lessons learned. AJR Am J Roentgenol. 2018;211(6):1381–9.
Uhlig J, Strauss A, Rucker G, et al. Partial nephrectomy versus ablative techniques for small renal masses: a systematic review and network meta-analysis. Eur Radiol. 2019;29(3):1293–307.
Wells SA, Wheeler KM, Mithqal A, Patel MS, Brace CL, Schenkman NS. Percutaneous microwave ablation of T1a and T1b renal cell carcinoma: short-term efficacy and complications with emphasis on tumor complexity and single session treatment. Abdom Radiol (NY). 2016;41(6):1203–11.
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
W. Prevoo was proctor for the Emprint system during the study period. The rest of the authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed Consent
For this type of study, formal consent is not required.
Consent for Publication
Consent for publication was obtained for every individual person’s data included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aarts, B.M., Prevoo, W., Meier, M.A.J. et al. Percutaneous Microwave Ablation of Histologically Proven T1 Renal Cell Carcinoma. Cardiovasc Intervent Radiol 43, 1025–1033 (2020). https://doi.org/10.1007/s00270-020-02423-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-020-02423-7