Abstract
The present study evaluated the anti-amnesic activity of 1-(7-chloroquinolin-4-yl)-5-methyl-N-phenyl-1H-1,2,3-triazole-4-carboxamide (QTCA-1) against scopolamine (SCO)-induced amnesia in mice. It was evaluated cholinergic dysfunction, oxidative stress and Na+/K+-ATPase activity in cerebral cortex and hippocampus of mice. Male Swiss mice were treated with QTCA-1 (10 mg/kg, intragastrically (i.g.), daily) for nine days. Thirty minutes after the treatment with compound, the animals received a injection of SCO (0.4 mg/kg, intraperitoneally (i.p.)). Mice were submitted to the behavioral tasks 30 min after injection of SCO (Barnes maze, open-field, object recognition and location, and step-down inhibitory avoidance tasks) during nine days. In day 9, cerebral cortex and hippocampus of mice were removed to determine the thiobarbituric acid reactive species (TBARS) levels, and catalase (CAT), Na+/K+-ATPase and acetylcholinesterase (AChE) activities. SCO caused amnesia in mice for changing in step-down inhibitory avoidance, Barnes maze, and object recognition and object location tasks. QTCA-1 treatment attenuated the behavioral changes caused by SCO. Moreover, SCO increased AChE and CAT activities, decreased Na+/K+-ATPase activity and increased TBARS levels in the cerebral structures of mice. QTCA-1 protected against these brain changes. In conclusion, QTCA-1 had anti-amnesic action in the experimental model used in the present study, through the anticholinesterase effect, modulation of Na+/K+-ATPase activity and antioxidant action.
Similar content being viewed by others
Introduction
Dementia is a state of cognitive decline, associated with psychiatric and behavioral disturbances (Ritchie et al. 2015). This syndrome is considered one of the major causes of morbidity and mortality among older people (Van de Vorst et al. 2015). In 2015, dementia affected 47 million people worldwide (or roughly 5% of the world’s elderly population), a figure that is predicted to increase to 75 million in 2030 and 132 million by 2050 (WHO 2017). Recent reviews estimate that globally nearly 9.9 million people develop dementia each year (WHO 2017).
Dementias may be difficult to clinically diagnose because of their multifactorial causes and overlapping symptoms with various neurological disorders, such as Alzheimer’s disease (AD), resulting in inconsistent clinical presentation and diagnostic challenges (Nelson et al. 2011; Puri et al. 2014). Many of neurological disorders associated with the dementia are often related to deficiencies in cerebral cholinergic neurotransmission (Lobo et al. 2000). Indeed, the blockade of muscarinic acetylcholine (ACh) receptors interrupts the learning and memory functions, given that ACh is an important neurotransmitter involved in regulating of cognitive functions (Kumar et al. 2015). A reduction in the ACh levels in cerebro-spinal fluid of AD patients is correlated inversely with the severity of dementia (Lin et al. 2016). Moreover, decreased ACh concentration in brain also results in a diminished ability to learn and form new memories (Lin et al. 2016). Acetylcholinesterase (AChE) is an important regulatory enzyme that modulates cholinergic synapses through the hydrolysis of ACh (Kumar et al. 2015).
Besides the cholinergic system, oxidative stress is another well-known causative factor in the pathogenesis of neurological disorders related to dementia (Cruz et al. 2002). Studies have shown that oxidative stress is related to cognitive impairments (Tao et al. 2015), being also connected to neuronal loss in brain (Azman et al. 2015). In addition, Na+/K+-ATPase is a potent neuroprotective modulator against neurological disorders associated dementia, given that oxidative stress modulates memory process through of Na+/K+-ATPase activity (Zhang et al. 2013).
Clinically, primary treatments for most forms of dementia in AD approved by Food and Drug Administration (FDA) are AChE inhibitors, such as donepezil, galantamine and rivastigmine (Zemek et al. 2014). However, the usual therapies for dementia treatment in AD provide symptomatic relief (Zemek et al. 2014). These treatments are effective in the early stages of disease and they presented some limitations, such as low efficacy and adverse effects for the long-term use (Libro et al. 2016). Consequently, the development of effective strategies that help to prevent age- and disease-related worsening of brain structure and function may thus provide significant benefits for society and health-care systems.
Several researches have suggested that the quinoline moiety is the pharmacophore of many anti-AD drugs, such as tacrine, clioquinol, methylene blue, berberine derivatives and PMS1339 (Freeman and Dawson 1991; Mancino et al. 2009; Oz et al. 2009; Jiang et al. 2011). In this context, our research group has dedicated attention to study the effect of quinoline derivatives in disorders affecting the central nervous system. Among the quinoline derivatives, 1-(7-chloroquinolin-4-yl)-5-methyl-N-phenyl-1H-1,2,3-triazole-4-carboxamide (QTCA-1) showed anticonvulsant activity, mainly due to its lipophilic nature, being cerebral tissue the target of this compound (Wilhelm et al. 2014).
Therefore, the present study evaluated the anti-amnesic activity of QTCA-1 against scopolamine (SCO)-induced amnesia in mice. The effect of QTCA-1 was evaluated on SCO-induced cholinergic dysfunction in terms of AChE activity in hippocampus and cerebral cortex of mice. Moreover, oxidative stress and Na+/K+-ATPase activity were estimated in cerebral structures of mice as a plausible mechanism of action for anti-amnesic activity of QTCA-1 in such condition.
Material and methods
Chemicals
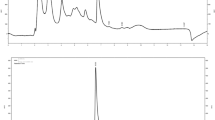
QTCA-1 (Fig. 1) was prepared according to the literature method (Wilhelm et al. 2014). Analysis of the 1H nuclear magnetic resonance (NMR) and 13C NMR spectra showed analytical and spectroscopic data in full agreement with its assigned structure. QTCA-1 was dissolved in canola oil. (−) Scopolamine hydro bromide, 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) and thiobarbituric acid (TBA) were purchased from the Sigma Chemical Co. (St Louis, Missouri, USA). (−) Scopolamine hydro bromide was dissolved in saline 0.9%. All other chemicals were of analytical grade and obtained from standard commercial suppliers.
Animals and ethical approval
Male adult Swiss mice (25–35 g) from a local breeding colony were used. Animals were kept on a separate room, on a 12 h light/dark cycle, at a temperature of 22 ± 2 °C, with free access to food and water. All animal experiments in the present study were approved by the Committee on Care and Use of Experimental Animal Resources of Federal University of Pelotas, Brazil (CEEA number 1974–2016) and in accordance with the guide of Brazilian National Animal Care Ethical Council (CONCEA), which is based on National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (Publication no. 85–23, revised 1985).
Experimental protocol
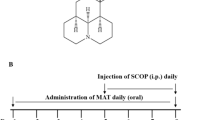
Mice were randomly divided into 4 experimental groups (7 animals/group, total of 28 animals): Group I (control) received canola oil + saline, group II (QTCA-1) received QTCA-1 + saline, group III (SCO) received canola oil + SCO, and group IV pre (QTCA-1 + SCO) received QTCA-1 + SCO. Initially, groups I and III received, intragastrically (i.g.) via gavage, canola oil (10 ml/kg), while groups II and IV were treated, i.g. via gavage, with QTCA-1 (10 mg/kg). Thirty minutes after treatments, groups III and IV received SCO (0.4 mg/kg, intraperitoneally (i.p.)) (Pahaye et al. 2017) and groups I and II received saline 0.9% (5 ml/kg, i.p.). Treatments were performed daily during the 9-day period. Thirty minutes after SCO or saline injections, animals were subjected to the behavioral tests. In day 9, animals were euthanized, and cerebral cortex and hippocampus were removed for ex vivo experiments (Fig. 2).
Scheme of experimental protocol. Thirty minutes before initiating intragastric (i.g.) treatments, mice received scopolamine (SCO) or saline, both intraperitoneally (i.p.). I.g. treatments and i.p. induction were performed every day, until the end of the experimental protocol. From the first day of the experimental protocol, the animals were submitted to the Barnes maze, open-field, object recognition, object location, step-down inhibitory avoidance tasks. On the ninth day, mice were euthanized
The choice of QTCA-1 dose was based on other studies of memory decline induced by SCO (Fukuda et al. 2019; da Silva et al. 2017; Eduviere et al. 2015). In these studies, different drugs exerted pharmacological effect at the dose of 10 mg/kg. Thus, considering the potential of these drugs in improving the memory decline and the necessity to reduce the number of animals used in experiments, based on humanitarian principles of animal experimentation or principle of the 3Rs (Russell and Burch 1959), a curve of dose-response was not tested. We opted by the oral route of administration for QTCA-1 because of the advantages presented, such as, low total dose, low gastrointestinal side effects, reduced dosing frequency, good patient acceptance and compliance, less fluctuation at plasma drug levels, more uniform drug effect, improved efficacy/safety ratio and low cost-effectiveness (Mignani et al. 2013). This has been the route of the preferred for the several authors that search alternative treatments for amnesia (Martini et al. 2018; Souza et al. 2010; Nath et al. 2009). Since it is well described in the literature that intraperitoneal administration of SCO induces amnesia in animals, this route of administration was chosen for the present study (Ngoupaye et al. 2017; Pahaye et al. 2017; da Silva et al. 2017; Habiba et al. 2017).
Behavioral tests
Barnes maze task
Spatial learning and memory were accessed using Barnes maze task (Pompl et al. 1999), with minor adaptations (Souza et al. 2012). This behavioral test was performed from the first day of the experimental protocol. The Barnes maze consists of a flat and circular disk (69 cm diameter) with sixteen circular holes (4.5 cm diameter) at equal distances around the perimeter, and it elevates 50 cm above the floor. The escape box (13 × 29 × 14 cm) was localized under of one hole. Mice learn the location of escape box under hole, using spatial reference points fixed to the wall. Animals were trained in the maze Barnes on days 1, 2, 3 and 4 of experimental protocol. Training consisted of placing the animal in a black box and leaving it for a period of 1 min. Then, black box was placed in the center of the Barnes maze. Black box was removed and then started the training. Mice freely explored the maze to find the escape box. Maximum latency to find out escape box was 180 s. Each mouse was trained three times per day, with 10-min inter-trial interval, during four consecutive days. The latency to reach the escape box and number of wrong holes were measured in four training sessions. Twenty-four hours after the last day of training phase (day 5 of experimental protocol), a probe trial was performed. In the probe trial, each mouse was placed in maze center, it was measured the latency to find the escape box and checked number of holes visited.
Open-field test
The locomotor and exploratory behaviors were assessed in the open-field test (Walsh and Cummins 1976). The open-field was made of plywood and surrounded by 30 cm-high walls. The floor of the open-field, 45 cm long and 45 cm wide, was divided by masking tape markers into 9 squares (3 rows of 3). Each animal was placed individually at the center of the apparatus and observed for 4 min to record the locomotor (number of segments crossed with the four paws) and exploratory (number of time rearing on the hind limbs). Mice were habituated to the open-field for 3 days (on days 3, 4 and 5 of experimental protocol) after Barnes maze task.
Object recognition task
The object recognition task is used as a measure to evaluate the long-term (LTM) memories (Stangherlin et al. 2009). The object recognition task was performed in an open-field apparatus. On the day of the test (day 6 of the experimental protocol), each animal was submitted to a habituation session in the absence of objects for 5 min. Subsequently, four objects were used: A1, A2, and B. The A1 and A2 objects were two identical balls, the B object was an octagon. Each object had the following color pattern: blue, red and yellow. All objects were made of plastic material, measuring 10 × 10 cm (length×height). During the training, in the day 6 of the experimental protocol, the animals were placed in the arena containing two identical objects (objects A1 and A2) for 5 min. Exploration was defined when the animal directed its nose within 2 cm of the object while looking, sniffing, or touching it. The LTM was performed 24 h after training phase, in the day 7 of the experimental protocol, when mice were placed to explore a familiar object (A1) and a new object (B) for 5 min and the total time spent in exploring each object was determined. Data were expressed as a percentage of the exploratory preference and calculated as follows: Training = (A2 /(A1 + A2)) × 100; LTM = (B/(A1 + B)) × 100.
Object location task
The object location task was performed according to Dix and Aggleton (1999). This test is a hippocampal-dependent spatial memory task. The apparatus used for this test was the same open-field apparatus used in the object recognition task with the LTM objects (object A1 and object B). In this task, four hours after the LTM, object B was moved to a location that was diagonally opposite to object A1, and the mouse was left in the apparatus for 5 min of exploration. The time spent exploring the location of new and familiar objects was recorded. The object location test is realized in the day 7 of protocol experimental. Data were expressed as a percentage of the exploratory preference and calculated as follows: object location task = (B/(A1 + B)) × 100.
Step-down inhibitory avoidance task
Long-term memory was investigated using the step-down inhibitory avoidance task (Sakaguchi et al. 2006), with modifications in the intensity of electric shock and in the exposure time. Animals were trained on step-down inhibitory avoidance in day 8 of experimental protocol. During the training session, each mouse was placed on the platform. When it stepped down and placed its four paws on the grid floor, an electric shock (0.5 mA) was delivered for 2 s. The test (30 min after scopolamine or saline injection) was performed 24 h after training (in day 9). Each mouse was placed again on the platform and the step-down transfer latency time was recorded, but mice no received the aversive stimulus.
Ex vivo assays
In the day 9 of the experimental protocol, mice were anesthetized and euthanized with isoflurane and cerebral structures (cerebral cortex and hippocampus) were immediately removed. Cerebral cortex and hippocampus were separated and washed with cold saline solution (0.9%) to determine AChE, Na+/K+-ATPase, and catalase (CAT) activities, as well as, thiobarbituric acid reactive species (TBARS) levels.
Cerebral structures were homogenized in cold 50 mM Tris-HCl, pH 7.4 (1/10, weight/volume), centrifuged at 900 xg at 4 °C for 10 min and supernatants were used for determination of enzymes activities (Na+/K+-ATPase and CAT) and TBARS levels. Also, cerebral cortex and hippocampus of mice were homogenized in 0.25 M sucrose buffer (1/10, w/v), centrifuged at 900 xg at 4 °C for 10 min and supernatants were used for determination of AChE activity.
AChE activity
The activity of AChE was determined by a modified method by Ellman et al. (1961), using acetylthiocholine as substrate. An aliquot of the supernatant (protein of 2.8 mg/mL) was pre-incubated for 2 min at 25 °C in a medium containing 100 mM, potassium phosphate buffer, pH 7.5. Enzymatic reaction was initiated by adding DTNB (final concentration of 0.5 mM) and acetylthiocholine (final concentration of 0.8 mM). The rate of hydrolysis of acetylthiocholine iodide was measured at 412 nm through the release of the thiol compound, which when reacted with DTNB produces the color-forming compound thionitrobenzene (TNB). Results were expressed as μmol acetylthiocholine/h/mg protein.
Na+/K+ ATPase activity
Supernatant was mixed with 3 mM MgCl, 125 mM NaCl, 20 mM KCl and 50 mM Tris-HCl, pH 7.4. The reaction was initiated by the addition of 3 mM adenosine triphosphate (ATP). Control samples were carried out under the same conditions with the addition of 0.1 mM ouabain. Cerebral cortex and hippocampus protein extracts taken for the assay were 8 and 7 mg/ml, respectively. The samples were incubated at 37 °C for 30 min and incubation was stopped by adding trichloroacetic acid (10%) with 10 mM HgCl2. Na+/K+-ATPase activity was calculated by the difference between the two assays. Released inorganic phosphate (Pi) was measured according to the method described by Fiske and Subbarow (1925). Enzyme activity was expressed as nmol Pi/mg protein/min.
TBARS levels
TBARS levels were determined as described by Ohkawa et al. (1979) and used as lipid peroxidation measure. An aliquot of supernatant was added to the reaction mixture containing: 8.1% sodium dodecyl sulfate (SDS), 0.8% thiobarbituric acid and acetic acid buffer (pH 3.4). The system was incubated at 95 °C for 2 h. Absorbance was measured at 532 nm. Results were reported as nmol malondialdehyde (MDA)/mg protein.
CAT activity
Enzyme activity was assayed by the method of Aebi (1984), which involves monitoring the disappearance of H2O2 in the homogenate at 240 nm. Enzymatic reaction was initiated by adding of the supernatant and the substrate (H2O2) in a medium containing 50 mM potassium phosphate buffer, pH 7.0. The enzymatic activity was expressed as Unit (U) CAT/mg protein (1 U decomposes 1 μmol H2O2/min at pH 7 at 25 °C).
Protein quantification
The protein concentration was measured by the method of Bradford (1976), using bovine serum albumin as the standard.
Statistical analysis
Data are expressed as means ± standard error of the mean (SEM). The normality of data was evaluated by the D’Agostino and Pearson omnibus normality test. Statistical analysis was performed using one-way (for data of Barnes maze task in the test phase and for the other behavioral and biochemical tests) or two-way (for data of Barnes maze task in the training phase) ANOVA followed by the Newman-Keuls multiple comparisons test. Main effects are presented only when the higher second order interaction was non-significant. Values of p < 0.05 were considered statistically significant.
Results
Behavioral tests
Barnes maze task
In the training phase, the two-way analysis of the latency to find the escape box revealed the main effect of days (ANOVA: F3, 96 = 12.88, p < 0.0001) and treatments (ANOVA: F3, 96 = 6.689, p < 0.001). When the number of holes visited was evaluated in the training phase, the two-way analysis showed significant main effect of days (ANOVA: F3, 96 = 12.66, p < 0.0001) and treatments (ANOVA: F3, 96 = 3.492, p < 0.05). On the first, second and third days of training, no difference between the groups in latency to find the escape box and number of holes visited was observed (Fig. 3A and 4A, respectively).
Effect of 1-(7-chloroquinolin-4-yl)-5-methyl-N-phenyl-1H-1,2,3-triazole-4-carboxamide (QTCA-1) on scopolamine (SCO)-induced memory deficits in Barnes maze task on the latency to find the escape box (s) (3A) on training and (3B) on the test phases. Data are reported as mean ± standard error of the mean (S.E.M.) of seven animals per group. (***) denotes p < 0.001 and (****) denotes p < 0.0001 as compared to the control group; (###) denotes p < 0.001 and (####) denotes p < 0.0001 as compared to the SCO group (two-way analysis of variance/Newman-Keuls test for training and one-way analysis of variance/Newman-Keuls test for probe test)
Effect 1-(7-chloroquinolin-4-yl)-5-methyl-N-phenyl-1H-1,2,3-triazole-4-carboxamide (QTCA-1) on scopolamine (SCO)-induced memory deficits in Barnes maze task in the number of holes visited (4A) on training and (4B) on the test phases. Data are reported as mean ± standard error of the mean (S.E.M.) of seven animals per group. (***) denotes p < 0.001 and (****) denotes p < 0.0001 as compared to the control group; (###) denotes p < 0.001 and (####) denotes p < 0.0001 as compared to the SCO group (two-way analysis of variance/Newman-Keuls test for training and one-way analysis of variance/Newman-Keuls test for probe test)
On the fourth day of training, SCO injection significantly increased (around 102%) the latency to find the escape box (Fig. 3A) and number of holes visited (around 90%) (Fig. 4A). QTCA-1 treatment significantly protected against this increase, when compared to the control group (Fig. 3A and 4A, respectively).
In the probe test (day 5), SCO increased the latency time to find the escape box (around 166%), and the number of holes visited (around 87%), when compared with the control group (Fig. 3B and 4B, respectively). QTCA-1 attenuated the latency to find the escape box and number of holes visited increased by SCO (Fig. 3B and 4B, respectively) (ANOVA: F3, 24 = 15.64, p < 0.0001 for latency and ANOVA: F3, 24 = 24.42, p < 0.0001 for number of holes).
Open-field test
The one-way ANOVA followed by Newman-Keuls test demonstrated that treatments did not cause any significant change in the number of crossings in days 1 (ANOVA: F3,24 = 1.956, p > 0.05), 2 (ANOVA: F 3, 24 = 2.902, p > 0.05) and 3 (ANOVA: F3,24 = 2.431, p > 0.05) (Table 1). One-way ANOVA for the number of rearing in first day (ANOVA: F3,24 = 1.806, p > 0.05), second day (ANOVA: F3,24 = 1.800, p > 0.05) and third day (ANOVA: F3,24 = 0.6916, p > 0.05) revealed no significant difference (Table 1).
Object recognition task
In the training phase of the object recognition task, there was no difference in the exploratory preference of objects among groups (ANOVA: F3, 24 = 1.078, p > 0.05) (Fig. 5A). In the probe test, mice treated with SCO had a decrease (around 28% for LTM) in the exploratory preference of the new object, and QTCA-1 prevented this reduction (Fig. 5B) (ANOVA: F3, 24 = 8.216, p < 0.01). QTCA-1 alone did not change the exploratory preference for the new object in LTM (Fig. 5B).
Effects of 1-(7-chloroquinolin-4-yl)-5-methyl-N-phenyl-1H-1,2,3-triazole-4-carboxamide (QTCA-1) on scopolamine (SCO)-induced memory deficits in the object recognition task during (5A) training phase, (5B) the long-term (LTM) memories and (5C) location memory. Data are reported as mean ± standard error of the mean (SEM) of seven animals per group. (*) denotes p < 0.05 and (**) denotes p < 0.01 as compared to the control group; (#) denotes p < 0.05, (##) denotes p < 0.01 and (###) denotes p < 0.001 as compared to the SCO group (one-way analysis of variance/Newman-Keuls test)
Object location task
For the object location task, mice injected with SCO had a reduction (around 33%) in the exploratory preference by the new object location and QTCA-1 significantly prevented this reduction, when compared to the control group (Fig. 5C) (ANOVA: F3, 24 = 4.508, p < 0.05). QTCA-1 alone did not change the exploratory preference for the new object location (Fig. 5C).
Step-down inhibitory avoidance
During the training session of the step-down inhibitory avoidance, there was no difference in the transfer latency time among groups (ANOVA: F3, 24 = 0.3553, p > 0.05) (Fig. 6). In the test phase, SCO decreased (around 70%) the transfer latency time and QTCA-1 significantly prevented this reduction, when compared to the control group (Fig. 6) (ANOVA: F3, 24 = 11.14, p < 0.0001). Mice treated only with QTCA-1 did not change the transfer latency time in the test phase (Fig. 6).
Effect 1-(7-chloroquinolin-4-yl)-5-methyl-N-phenyl-1H-1,2,3-triazole-4-carboxamide (QTCA-1) on scopolamine (SCO)-induced memory deficits in step-down inhibitory avoidance: latency (s) to fall from the platform in the training and test phases. Data are reported as mean ± standard error of the mean (SEM) of seven animals per group. (****) denotes p < 0.0001 as compared to the control group; (###) denotes p < 0.001 and (####) denotes p < 0.0001 as compared to the SCO group (one-way analysis of variance/Newman-Keuls test)
Ex vivo assays
AChE activity
Figure 7A and B illustrate the effects of treatments on AChE activity in hippocampus and cerebral cortex of mice, respectively. Results demonstrated that SCO increased the AChE activity in hippocampus (around 182%) and cerebral cortex (around 235%) of mice, when compared with the control group. QTCA-1 treatment significantly prevented the increase on the AChE activity in cerebral structures caused by SCO (Fig. 7A and B, respectively). QTCA-1 alone did not change the AChE activity in the hippocampus and cerebral cortex (Fig. 7A and B, respectively) (ANOVA: F3, 24 = 20.78, p < 0.0001 for hippocampus and ANOVA: F3, 24 = 7.555, p < 0.001 for cerebral cortex).
Effect of 1-(7-chloroquinolin-4-yl)-5-methyl-N-phenyl-1H-1,2,3-triazole-4-carboxamide (QTCA-1) on acetylcholinesterase (AChE) activity in (7A) hippocampus and (7B) cerebral cortex of mice after induction with scopolamine (SCO). Data are reported as mean ± standard error of the mean (SEM) of seven animals per group. (**) denotes p < 0.01, (***) denotes p < 0.001 and (****) denotes p < 0.0001 as compared to the control group; (##) denotes p < 0.01 (###) denotes p < 0.001 and (####) denotes p < 0.0001 as compared to the SCO group (one-way analysis of variance/Newman-Keuls test)
Na+/K+-ATPase activity
SCO induced a reduction on the Na+/K+ ATPase activity (around 57%) in hippocampus (Fig. 8A) and cerebral cortex (around 76%) (Fig. 8B) of mice. QTCA-1 treatment protected against the reduction in the Na+/K+-ATPase activity caused by SCO in both cerebral structures of mice (Fig. 8A for hippocampus and 8B for cerebral cortex). Treatment only with QTCA-1 did not modify the Na+/K+-ATPase activity in hippocampus and cerebral cortex of mice (Fig. 8A and B, respectively) (ANOVA: F3, 24 = 6.307, p < 0.01 for hippocampus and ANOVA: F3, 24 = 4.552, p < 0.05 for cerebral cortex).
Effect of 1-(7-chloroquinolin-4-yl)-5-methyl-N-phenyl-1H-1,2,3-triazole-4-carboxamide (QTCA-1) on Na+/K+-ATPase activity in (8A) hippocampus and (8B) cerebral cortex of mice after induction with scopolamine (SCO). Data are reported as mean ± standard error of the mean (SEM) of seven animals per group. (*) denotes p < 0.05 as compared to the control group; (#) denotes p < 0.05 and (##) denotes p < 0.01 as compared to the SCO group (one-way analysis of variance/Newman-Keuls test)
TBARS levels
SCO increased the TBARS levels in the hippocampus (around 67%) and in the cerebral cortex (around 83%) (Fig. 9A and B, respectively). QTCA-1 treatment protected against the increase caused by SCO in both cerebral structures (Fig. 9A and B). QTCA-1 alone did not change the TBARS levels in the hippocampus and cerebral cortex (Fig. 9A and B, respectively) (ANOVA: F3, 24 = 14.84, p < 0.0001 for hippocampus and ANOVA: F3, 24 = 11.69, p < 0.0001 for cerebral cortex).
Effect of 1-(7-chloroquinolin-4-yl)-5-methyl-N-phenyl-1H-1,2,3-triazole-4-carboxamide (QTCA-1) on thiobarbituric acid reactive species (TBARS) levels in (9A) hippocampus and (9B) cerebral cortex of mice after induction with scopolamine (SCO). Data are reported as mean ± standard error of the mean (SEM) of seven animals per group. (*) denotes p < 0.05, (**) denotes p < 0.01 and (***) denotes p < 0.001 as compared to the control group, (##) denotes p < 0.01, (###) denotes p < 0.001, and (####) denotes p < 0.0001 as compared to the SCO group (one-way analysis of variance/Newman-Keuls test)
CAT activity
Figure 10A and B illustrate the effects of treatments on the CAT activity in hippocampus and cerebral cortex of mice, respectively. Results demonstrated that SCO increased the CAT activity in hippocampus (around 178%) and the cerebral cortex (around 132%) of mice, when compared to the control group. QTCA-1 treatment significantly prevented the increase in the CAT activity in hippocampus and cerebral cortex caused by SCO (Fig. 10A and B, respectively). Treatment only with QTCA-1 did not modify the CAT activity in hippocampus and cerebral cortex of mice (Fig. 10A and B, respectively) (ANOVA: F3, 24 = 4.035, p < 0.05 for hippocampus and ANOVA: F3, 24 = 4.506, p < 0.05 for cerebral cortex).
Effect 1-(7-chloroquinolin-4-yl)-5-methyl-N-phenyl-1H-1,2,3-triazole-4-carboxamide (QTCA-1) in the catalase (CAT) activity in (10A) hippocampus and (10B) cerebral cortex of mice after induction with scopolamine (SCO). Data are reported as mean ± standard error of the mean (SEM) of seven animals per group. (*) denotes p < 0.05 as compared to the control group; (#) denotes p < 0.05 as compared to the SCO group (one-way analysis of variance/Newman-Keuls test)
Discussion
The present study demonstrated, for the first time, that the administration of QTCA-1 for 9 days mitigated amnesia in SCO-challenged mice. Moreover, QTCA-1 attenuated SCO-induced cholinergic dysfunction, oxidative stress and Na+/K+-ATPase activity in hippocampus and cerebral cortex of mice and it did not cause changes in locomotor and exploratory activity in the open-field test. These results may implicate the fact that QTCA-1, could be a potential candidate in the management of AD and other neurological disorders associated with dementia.
Cholinergic dysfunction is one of the responsible factors for amnesia in the pathophysiology of AD (Puri et al. 2014; Kumar et al. 2015). To assess the amnesia in the SCO mouse model, four types of cognitive behavioral tests were conducted. Similar to previous findings, as expected, SCO caused amnesia in mice in terms of changes in step-down inhibitory avoidance, Barnes maze, and object recognition and object location tasks. SCO exhibits amnesia through antagonistic activity on muscarinic receptors in the brain of experimental animals (da Silva et al. 2017; Pahaye et al. 2017).
Blockage of cholinergic neurons by SCO is widely used in the screening of new drugs for the treatment of dementia in neurodegenerative disorders (Chen et al. 2014; Weon et al. 2016; da Silva et al. 2017; Kim et al. 2018). In this context, the same four types of cognitive behavioral tests were performed as screening methods to show anti-amnesic action of QTCA-1. QTCA-1 treatment attenuated spatial learning and reference memory (as shown by reducing the latency to find the escape box and number of holes visited in the Barnes maze task), non-spatial aversive long-term memory (as evidenced by prolonging the latency time in the step-down inhibitory avoidance), LTM (as demonstrated by increasing the exploratory preference for the new object in the object recognition task) and spatial memory (as evidenced by increasing the exploratory preference for the new object location in the object location task) induced by SCO in mice. Our results highlight that QTCA-1 has an anti-amnesic action.
Here, we evaluated the exploratory and locomotory activities of the animals in the open field test for 3 days in order to demonstrate that the treatments did not influence in these parameters and to habituate the animals in this environment for the object recognition task. The exploratory and locomotory activities are important for the evaluated tests. Indeed, in the present study the observed effects were not related to the changes in spontaneous locomotor and exploratory activities of mice in the open field test, so treatments were devoid of motor stimulant properties. In addition, the animals presented a good response in the exploratory preference.
Furthermore, SCO induces a cerebral cholinergic dysfunction by decreasing level of ACh and increasing activity of AChE (Guo et al. 2016). Our results showed that SCO increased AChE activity in cerebral cortex and hippocampus of mice. Importantly, QTCA-1 administration protected SCO-induced cholinergic dysfunction in both cerebral structures of animals in the present study. In this context, anticholinesterase effect of QCTA-1 could be a mechanism by which the compound prevented behavioral changes caused by SCO. Indeed, inhibition in the AChE activity is proofed to be an attractive strategy for the treatment of dementia (Parent and Baxter 2004; Kim et al. 2018), given that memory disorders typically exhibit cholinergic deficits (abnormally elevated AChE activity and reduced ACh levels). Therefore, QTCA-1 may proves to be a therapeutic strategy for some neurodegenerative disorders not yet treated, like AD.
In addition to cholinergic dysfunction, the increase in oxidative stress with age is the main risk factor for amnesia in neurodegenerative disorders, mainly AD (Niedzielska et al. 2016; Ferreira-Vieira et al. 2016). The state of brain after SCO injection can be determined by the state of oxidative stress. We determined the redox status of brain by measuring the levels of TBARS and activity of CAT. In the present study, we reported that there was an elevation in the levels of TBARS and in the CAT activity in cerebral cortex and hippocampus of mice caused by SCO. Indeed, SCO has a robust effect on the brain; that is, the redox state imbalance of the cerebral cortex and hippocampus is aggravated (Balaban et al. 2016; Pahaye et al. 2017).
In this context, it is well documented that antioxidants molecules can be promising therapeutic strategies for treating amnesia and dementia in some neurodegenerative disorders (Mattson et al. 1999; Pinz et al. 2018; Sambon et al. 2019; Mazumder et al. 2019). Here, we verified that treatment with QTCA-1 protected against increasing TBARS levels and CAT activity in cerebral structures of mice injected with SCO.
Na+/K+-ATPase is an important neuromodulator against AD, given that the deficiency of Na+/K+-ATPase causes learning and memory deficits (Zhang et al. 2013). In this study, we found a reduction in the Na+/K+-ATPase activity in cerebral cortex and hippocampus of animals treated with SCO. The reduction in the enzyme activity by SCO has a central role in memory process (Gutierres et al. 2014; da Silva et al. 2017) and pathogenesis of neurodegenerative diseases, like AD (Gutierres et al. 2014). Thus, we can relate the decrease in Na+/K+-ATPase activity with the amnesia caused by SCO.
In line with this, QTCA-1 protected from the reduction in Na+/K+-ATPase activity induced by SCO in cerebral structures of mice. In this way, this finding indicates that anti-amnesic action of compound could be related with protective effect in Na+/K+-ATPase activity. In accordance, Ali and Arafa (2011) reported an increase in the Na+/K+-ATPase activity by anti-amnesic drugs. Furthermore, antioxidant effect of QTCA-1 could be involved in the beneficial effect of compound on the Na+/K+-ATPase activity, because it has been established that oxidative stress modulates memory process in AD through of Na+/K+-ATPase activity (Zhang et al. 2013).
In this sense, this study demonstrated that QTCA-1 had anti-amnesic action by protecting the AChE and Na+/K+-ATPase activities changed by SCO, and also by its antioxidant effect. Therefore, this compound has been shown to be a pharmacological alternative for the treatment of dementia related to some neurodegenerative disorders, mainly AD. Moreover, more studies are needed to elucidate the other mechanisms of QTCA-1, mainly to deepen its action on the cholinergic system.
Although promising results have been obtained here, it is important to note that one of the limitations of this study is the evaluation of QTCA-1 using only the dose of 10 mg/kg. A dose–response curve could be evaluated, but because it was necessary to reduce the number of animals used in experiments, based on humanitarian principles of animal experimentation or principle of the 3Rs (Russell and Burch 1959), it was not done. In addition, it is important to highlight that due to the small amount of samples of cerebral cortex and hippocampus other assays can not performed. Also, mouse model used is not a disease model with permanent amnesia. Thus, further studies are necessary to evaluate the pharmacological potential of QTCA-1 using different model of induction of amnesia. Although QTCA-1 exerts an important pharmacological action, the evaluation of toxicological effects of acute and repeated administrations of QTCA-1 should be elucidated in further studies.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Ali EHA, Arafa NMS (2011) Comparative protective action of curcumin, memantine and diclofenac against scopolamine-induced memory dysfunction. Fitoterapia 82:601–608. https://doi.org/10.1016/j.fitote.2011.01.016
Azman KF, Zakaria R, Abdul-Aziz C, Othman Z, Al-Rahbi B (2015) Tualang honey improves memory performance and decreases depressive-like behavior in rats exposed to loud noise stress. Noise Health 17:83–89. https://doi.org/10.4103/1463-1741.153388
Balaban H, Nazıroglu M, Demirci K, Övey IS (2016) The protective role of selenium on scopolamine-induced memory impairment, oxidative stress, and apoptosis in aged rats: the involvement of TRPM2 and TRPV1 channels. Mol Neurobiol 54:2852–2868. https://doi.org/10.1007/s12035-016-9835-0
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Chen W, Cheng X, Chen J, Yi X, Nie D, Sun X (2014) Lycium barbarum polysaccharides prevent memory and neurogenesis impairments in scopolamine-treated rats. PLoS One 9(2):e88076. https://doi.org/10.1371/journal.pone.0088076
Cruz R, Almaguer MW, Bergado RJ (2002) Glutathione in cognitive function and neurodegeneration. Rev Neurol 36:877–886. https://doi.org/10.33588/rn.3609.2002395
da Silva FD, Pinz MP, de Oliveira RL, Rodrigues KC, Ianiski FR, Bassaco MM, Silveira CC, Jesse CR, Roman SS, Wilhelm EA, Luchese C (2017) Organosulfur compound protects against memory decline induced by scopolamine through modulation of oxidative stress and Na+/K+ ATPase activity in mice. Metab Brain Dis 6:1819–1828. https://doi.org/10.1007/s11011-017-0067-4
Dix SL, Aggleton JP (1999) Extending the spontaneous preference test of recognition:evidence of object location and object-context recognition. Behav Brain Res 99:191–200. https://doi.org/10.1016/S0166-4328(98)00079-5
Eduviere AT, Umukoro S, Aderibigbe AO, Ajayi AM, Adewole FA (2015) Methyl jasmonate enhances memory performance through inhibition of oxidative stress and acetylcholinesterase activity in mice. Life Sci 132:20–26. https://doi.org/10.1016/j.lfs.2015.04.007
Ellman GL, Courtney KD, Andres JV, Featherstone MR (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Ferreira-Vieira HT, Guimaraes IM, Silva FR, Ribeiro FM (2016) Alzheimer's disease: targeting the cholinergic system. Curr Neuropharmacol 14:101–115. https://doi.org/10.2174/1570159X13666150716165726
Fiske CH, Subbarow YJ (1925) The calorimetic determination of phosphorus. Biol Chem 66:375–381
Freeman SE, Dawson RM (1991) Tacrine: a pharmacological review. Prog Neurobiol 36:255–257. https://doi.org/10.1016/0301-0082(91)90002-I
Fukuda T, Ayabe T, Ohya R, Ano Y (2019) Matured hop bitter acids improve spatial working and object recognition memory via nicotinic acetylcholine receptors. Psychopharmacology 236(9):2847–2854. https://doi.org/10.1007/s00213-019-05263-7
Guo C, Shen J, Meng Z, Yang X, Li F (2016) Neuroprotective effects of polygalacic acid on scopolamine-induced memory deficits in mice. Phytomedicine 23:149–155. https://doi.org/10.1016/j.phymed.2015.12.009
Gutierres JM, Carvalho FB, Schetinger MRC, Agostinho P, MariscoPC VJM, Spanevello R (2014) Neuroprotective effect of anthocyanins on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia in rats. Int J Dev Neurosci 33:88–97. https://doi.org/10.1016/j.ijdevneu.2013.12.006
Habiba R, Aamra M, Touqeer A (2017) Title: role of cholinergic receptors in memory retrieval. Depends on Gender and Age of Memory Behav Brain Res 331:233–240. https://doi.org/10.1016/j.bbr.2017.05.017
Jiang HL, Wang X, Huang L, Luo Z, Su T, Ding K, Li X (2011) Benzenediol-berberine hybrids: multifunctional agents for Alzheimer's disease. Bioorg Med Chem 19:7228–7235. https://doi.org/10.1016/j.bmc.2011.09.040
Kim MS, Lee DY, Lee J, Kim HW, Sung SH, Han JS, Jeon WK (2018) Terminalia chebula extract prevents scopolamine-induced amnesia via cholinergic modulation and anti-oxidative effects in mice. BMC Complement Altern Med 18:1–11. https://doi.org/10.1186/s12906-018-2212-y
Kumar A, Singh A, Ekavali (2015) A review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacol Rep 67:195–203. https://doi.org/10.1016/j.pharep.2014.09.004
Libro RGS, Rajan ST, Bramanti P, Mazzon E (2016) Natural phytochemicals in the treatment and prevention of dementia: an overview. Molecules 21:1–38. https://doi.org/10.3390/molecules21040518
Lin J, Ling H, Jie Y, Siying X, Jialing W, Jinrong Z, Xiaojun Y, Wei C, Shan H, Qinwen W (2016) Fucoxanthin, a marine carotenoid, reverses scopolamine-induced cognitive impairments in mice and inhibits acetylcholinesterase in vitro. Mar Drugs 14:1–17. https://doi.org/10.3390/md14040067
Lobo A, Launer LJ, Fratiglioni L, Andersen K, Di Carlo A, Breteler MMB, Soininen H (2000) Prevalence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts. Neurology 54:4–9. https://doi.org/10.1186/1471-2377-9-55
Mancino AM, Hindo SS, Kochi A, Lim MH (2009) Effects of clioquinol on metal- triggered amyloid-b aggregation revisited. Inorg Chem 48:9596–9598. https://doi.org/10.1021/ic9014256
Martini F, Pesarico AP, Brüning CA, Zeni G, Nogueira CW (2018) Ebselen inhibits the activity of acetylcholinesterase globular isoform G4 in vitro and attenuates scopolamine-induced amnesia in mice. J Cell Biochem 119:5598–5608. https://doi.org/10.1002/jcb.26731
Mattson MP, Pedersen WA, Duan W, Culmsee C, Camandola S (1999) Cellular and molecular mechanisms underlying perturbed energy metabolism and neuronal degeneration in Alzheimer’s and Parkinson’s diseases. Ann N Y Acad Sci 893:154–175. https://doi.org/10.1111/j.1749-6632.1999.tb07824.x
Mazumder MK, Choudhury S, Borah A (2019) An in silico investigation on the inhibitory potential of the constituents of pomegranate juice on antioxidant defense mechanism: relevance to neurodegenerative diseases. IBRO Rep 6:153–159. https://doi.org/10.1016/j.ibror.2019.05.003
Mignani S, El Kazzouli S, Bousmina M, Majoral JP (2013) Expand classical drug administration ways by emerging routes using dendrimer drug delivery systems: a concise overview. Adv Drug Deliv Rev 10:1316–1330. https://doi.org/10.1016/j.addr.2013.01.001
Nath C, Agrawal R, Tyagi E, Saxena G (2009) Cholinergic influence on memory stages: a study on scopolamine amnesic mice. Indian J Pharmacol 41:192–196. https://doi.org/10.4103/0253-7613.56072
Nelson PT, Head E, Schmitt FA, Davis PR, Neltner JH, Jicha GA, Abner EL, Smith CD, Van Eldik LJ, Kryscio RJ, Scheff SW (2011) Alzheimer’s disease is not “brain aging”: neuropathological, genetic, and epidemiological human studies. Acta Neuropathol 121:571–587. https://doi.org/10.1007/s00401-011-0826-y
Ngoupaye GT, Pahaye DB, Ngondi J, Moto FCO, Bum EN (2017) Gladiolus dalenii lyophilisate reverses scopolamineinduced amnesia and reduces oxidative stress in rat brain. Biomed Pharmacother 91:350–357. https://doi.org/10.1016/j.biopha.2017.04.061
Niedzielska E, Smaga I, Gawlik M, Moniczewski A, Stankowicz P, Pera J, Filip M (2016) Oxidative stress in neurodegenerative diseases. Mol Neurobiol 53:4094–4125. https://doi.org/10.1007/s12035-015-9337-5
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Oz M, Lorke DE, Petroianu GA (2009) Methylene blue and Alzheimer's disease. Biochem Pharmacol 78:927–932. https://doi.org/10.1016/j.bcp.2009
Pahaye DB, Bum EN, Taïwé GS, Ngoupaye GT, Sidiki S, Moto FCO, Kouemou N, Njapdounke NSJK, Nkantchoua G, Kandeda O, Mairaira V, Ojong JL (2017) Neuroprotective and antiamnesic effects of mitragyna inermis willd (Rubiaceae) on scopolamine-induced memory impairment in mice. Behav Neurol 2017:5952897. https://doi.org/10.1155/2017/595289
Parent MB, Baxter MG (2004) Septohippocampal acetylcholine: involved in but not necessary for learning and memory? Learn Mem 11:9–20. https://doi.org/10.1101/lm.69104
Pinz MP, Reis AS, Vogt AG, Krüger R, Alves D, Jesse CR, Roman SS, Soares MP, Wilhelm EA, Luchese C (2018) Current advances of pharmacological properties of 7-chloro-4-(phenylselanyl) quinoline: prevention of cognitive deficit and anxiety in Alzheimer’s disease model. Biomed Pharmacother 105:1006–1014. https://doi.org/10.1016/j.biopha.2018.06.049
Pompl PN, Mullan MJ, Bjugstad K, Arendash GW (1999) Adaptation of the circular platform spatial memory task for mice: use in detecting cognitive impairment in the APP(SW) transgenic mouse model for Alzheimer’s disease. J Neurosci Methods 87:87–95. https://doi.org/10.1016/S0165-0270(98)00169-1
Puri A, Srivastava P, Pandey P, Yadav RS, Bhatt PC (2014) Scopolamine induced behavioral and biochemical modifications and protective effect of Celastrus paniculatous and Angelica glauca in rats. Int J Nutr Pharmacol Neurol Dis 4:158–169. https://doi.org/10.4103/2231-0738.132675
Ritchie CW, Terrera GM, Quinn TJ (2015) Dementia trials and dementia tribulations: methodological and analytical challenges in dementia research. Alzheimers Res Ther 7:31–11. https://doi.org/10.1186/s13195-015-0113-6
Russell WMS, Burch RL (1959) The principles of humane experimental technique. Methuen, London
Sakaguchi M, Koseki M, Wakamatsu M, Matsumura E (2006) Effects of systemic administration of beta-casomorphin-5 on learning and memory in mice. Eur J Pharmacol 530:81–87. https://doi.org/10.1016/j.ejphar.2005.11.014
Sambon M, Napp A, Demelenne A, Vignisse J, Wins P, Fillet M, Bettendorff L (2019) Thiamine and benfotiamine protect neuroblastoma cells against paraquat and β-amyloid toxicity by a coenzyme-independent mechanism. Heliyon. 5:e01710. https://doi.org/10.1016/j.heliyon.2019.e01710
Souza MA, Magni DV, Guerra GP, Oliveira MS, Furian AF, Pereira L, Marquez SV, Ferreira J, Fighera MR, Roves LFF (2012) Involvement of hippocampal CAMKII/CREB signaling in the spatial memory retention induced by creatine. Amino Acids 43:2491–2503. https://doi.org/10.1007/s00726-012-1329-4
Souza ACG, Brüning CA, Leite MR, Zeni G, Nogueira CW (2010) Diphenyl diselenide improves scopolamine-induced memory impairment in mice. Behav Pharmacol 21:556–562. https://doi.org/10.1097/FBP.0b013e32833befcf
Stangherlin EC, Rocha JBT, Nogueira CW (2009) Diphenyl ditelluride impairs short term memory and alters neurochemical parameters in young rats. Pharmacol Biochem Behav 91:430–435. https://doi.org/10.1016/j.pbb.2008.08.020
Tao S, Liu L, Shi L, Li X, Shen P, Xun Q, Guo X, Yu Z, Wang J (2015) Spatial learning and memory deficits in young adult mice exposed to a brief intense noise at postnatal age. J Otol 10:21–28. https://doi.org/10.1016/j.joto.2015.07.001
Van de Vorst IE, Vaartjes I, Sinnecker LF, Beks LJM, Bots ML, Koel HL (2015) The validity of national hospital discharge register data on dementia: a comparative analysis using clinical data from a university medical Centre. Neth J Med 73:69–75
Walsh RN, Cummins RA (1976) Open-field test: critical review. Psychol Bull 83:482–504
Weon JB, Jung YS, Ma CJ (2016) Cognitive-enhancing effect of Dianthus superbus Var. Longicalycinus on scopolamine-induced memory impairment in mice. Biomol Ther 24:298–304. https://doi.org/10.4062/biomolther.2015.083
Wilhelm EA, Machado NC, Pedroso AB, Goldani BS, Seus N, Moura S, Savegnago L, Jacob RG, Alves D (2014) Organocatalytic synthesis and evaluation of 7-chloroquinoline-1,2,3-triazoylcarboxamides as potential antinociceptive, anti-inflammatory and anticonvulsant agent. RSC Adv 04:41437–41445. https://doi.org/10.5935/0103-5053.20150239
World Health Organization (WHO) (2017). Global action plan on the public health response to dementia 2017–2025. Geneva: World Health Organization; 2017. Licence: CC BY-NC-SA 3.0 IGO
Zemek F, Drtinova L, Nepovimova E, Sepsova V, Korabecny J, Klimes J, Kuca K (2014) Outcomes of Alzheimer’s disease therapy with acetylcholinesterase inhibitors and memantine. Expert Opin Drug Saf 13:759–774. https://doi.org/10.1517/14740338.2014.914168
Zhang LN, Sun YJ, Pan S, Li JX, Qu YE, Li Y, Wang YL, Gao ZB (2013) Na+-K+-ATPase, a potent neuroprotective modulator against Alzheimer disease. Fundam Clin Pharmacol 27:96–103. https://doi.org/10.1111/fcp.12000
Acknowledgments
We are grateful for the financial support and scholarships from the Brazilian agencies CNPq (UNIVERSAL 408874/2016-3), FAPERGS (PRONEM 16/2551-0000240-1, PRONUPEQ 16/2551-0000526-5 and PqG 17/2551-0001013-2). CNPq is also acknowledged for the fellowship to C.L., D.A. and E.A.W. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível superior – Brasil (CAPES) - Finance Code 001.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Luchese, C., Vogt, A.G., Pinz, M.P. et al. Amnesia-ameliorative effect of a quinoline derivative through regulation of oxidative/cholinergic systems and Na+/K+-ATPase activity in mice. Metab Brain Dis 35, 589–600 (2020). https://doi.org/10.1007/s11011-020-00535-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-020-00535-0