Abstract
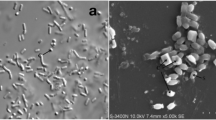
As a pathogenic bacterium, Bacillus thuringiensis (Bt) has become an alternative to chemical insecticides in commercial agricultural to control forestry pests and mosquitoes. To prevent pest resistance, many novel Bt strains have been isolated. Strain S3580-1 (WGS: VHPX0000000) used in this research and originally isolated from Hainan Qixianling National Forest Park (China) showed significant toxicity to Culex pipiens pallens. Here, using whole genome sequencing, assembly, and bioinformatics analysis, the predicted S3580-1CG_5163 (GenBank Accession No. MK124137) gene-encoded protein was found to share low homology with known toxins designated by the Bt toxin nomenclature system. It was considered to be an ETX/MTX2-type toxin and was designated Epp. Bioinformatics analysis showed that the predicted S3580-1CG_5163 gene-encoded protein Epp shared low identity with other known toxic protein sequences containing Cry-ETX/MTX conserved domains at the amino acid level, but significant similarity at the structural level. In addition, bioassays showed that Epp was toxic against Spodoptera litura (LC50 296.133 μg/mL; 95% FL 200.555–471.318 μg/mL) and Cx. pipiens pallens (LC50 322.193 μg/mL; 95% FL 238.217–477.243 μg/mL). On pathological observation, the peritrophic membrane of Cx. pipiens pallens larvae was degraded causing the midgut structure to become incomplete, resulting in larval death. Further bioassays are required to fully elucidate the insecticidal spectrum of the ETX/MTX2-type toxin Epp, and thereby provide future research directions.






Similar content being viewed by others
References
Adang MJ, Crickmore N, Jurat-Fuentes JL (2014) Diversity of Bacillus thuringiensis crystal toxins and mechanism of action. In: Dhadialla TS, Gill SS (eds) Insect midgut and insecticidal proteins, Advances in insect physiology, vol 47. Elsevier, Philadelphia, pp 39–88
Bokori-Brown M, Savva CG, Fernandes da Costa SP, Naylor CE, Basak AK, Titball RW (2011) Molecular basis of toxicity of Clostridium perfringens epsilon toxin. FEBS J 278:4589–4601
Bowen DJ, Chay CA, Flasinski S, Yin Y (2017) Novel insect inhibitory proteins. Monsanto Technology LLC, US Patent Application. 2017; No.A120170044568
Brown KL, Whiteley HR (1992) Molecular characterization of two novel crystal protein genes from Bacillus thuringiensis subsp. thompsoni. J Bacteriol 174:549–557
Baum JA, Sukuru UR, Penn SR, Meyer SE, Subbarao S, Shi X, Flasinski S, Heck GR, Brown RS, Clark TL (2012) Cotton plants expressing a Hemiptera-active Bacillus thuringiensis crystal protein impact the development and survival of Lygus hesperus (Hemiptera: Miridae) nymphs. J Econ Entomol 105:616–624
Liu Y, Wang Y, Shu C, Lin K, Song F, Bravo A, Soberón M, Zhang J (2018) Cry64Ba and Cry64Ca, two ETX/MTX2-type Bacillus thuringiensis insecticidal proteins active against Hemipteran pests. Appl Environ Microbiol 84:e01996–e01917
Moar W, Evans A, Kessenich CR, Baum JA, Bowen DJ, Edrington TC, Haas JA, Kouadio JK, Roberts JK, Silvanovich A (2017) The sequence, structural, and functional diversity within a protein family and implications for specificity and safety: the case for ETX_MTX2 insecticidal proteins. J Invertebr Pathol 142:50–59
Naimov S, Boncheva R, Karlova R, Dukiandjiev S, Minkov I, de Maagd RA (2008) Solubilization, activation, and insecticidal activity of Bacillus thuringiensis serovar thompsoni HD542 crystal proteins. Appl Environ Microbiol 74:7145–7151
Palma L, Muñoz D, Berry C, Murillo J, Caballero P (2014) Bacillus thuringiensis toxins: an overview of their biocidal activity. Toxins 6:3296–3325
Sanahuja G, Banakar R, Twyman RM, Capell T, Christou P (2011) Bacillus thuringiensis: a century of research, development and commercial applications. Plant Biotechnol J 9(3):283–300
Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler DR, Dean DH (1998) Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev 62(3):775–806
Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M (2006) In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc 1(6):2856–2860
Sun YJ, Zhao Q, Xia LQ, Ding XZ, Hu QF, Federici BA, Park HW (2013) Identification and characterization of three previously undescribed crystal proteins from Bacillus thuringiensis subsp. Jegathesan. Appl Environ Microbiol 79(11):3364–3370
Wirth MC (2010) Mosquito resistance to bacterial larvicidal toxins. Open Toxicol J 3:126–140
Yuan Z, Zhang Y, Cai Q, Liu EY (2000) High-level field resistance to Bacillus sphaericus C3-41 in Culex quinquefasciatus from southern China. Biocontrol Sci Tech 10:41–49
Zhang W, Crickmore N, George Z, Xie L, He YQ, Li Y, Tang JL, Tian L, Wang X, Fang X (2012) Characterization of a new highly mosquitocidal isolate of Bacillus thuringiensis–an alternative to Bti? J Invertebr Pathol 109:217–222
Acknowledgments
All of the intellectual property rights are owned by Hainan Institute of Tropical Agricultural Resources. The insect breeding and bioassay investigations were carried out in the Institute of Life Science of Jiyang College of Zhejiang A&F University. We thank Kate Fox, DPhil, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding
This work was supported by the Innovation Project of Guangxi Graduate Education (YCBZ2014006), the Key Laboratory of Subtropical Biomass Conservation and Utilization Key Laboratory Open Project (Novel Bt Strain Novel Toxin Genes and Its Protein Function Identification) (DD190043), and the Study Abroad Program for Excellent PhD Students of Guangxi Zhuang Autonomous Region (YZ).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Lucy Seldin
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, Y., Wu, Z., Zhang, J. et al. Bacillus thuringiensis novel toxin Epp is toxic to mosquitoes and prodenia litura larvae. Braz J Microbiol 51, 437–445 (2020). https://doi.org/10.1007/s42770-019-00194-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-019-00194-z