Abstract
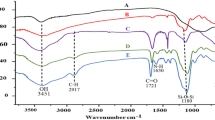
In this research, a novel magnetic cobalt ion imprinted adsorbent (Co(II)-MIIP) was synthesized by use of magnetic SBA-15 core-shell. It was functionalized by dithizone, and after identification by various techniques was used for removal of cobalt from aquatic systems. The uptake of cobalt proceeded very fast and achieved to equilibration within 5 min at which 74 mg g−1 of cobalt was adsorbed at pH = 8 with adsorbent dose of 0.15 g. The ion imprinted sorbent exhibited good selectivity towards cobalt ions. Separation and recovery of the used sorbent was carried out respectively by use of magnetic field and by use of HNO3 (0.1 M), and 85% of the initial capacity was obtained after seven 7 regeneration cycles. Different isotherm models, and error analysis were used to evaluate the experimental data. Thermodynamic, and kinetic evaluations showed that sorption process was endothermic, and described by second order kinetic model (R2 > 0.99). The equilibrium was established within five min.













Similar content being viewed by others
References
Yan X, Li QZ, Chai LY, Yang BT, Wang QW. Formation of a biological granular sludge – A facile and bioinspired proposal for improving sludge settling performance during heavy metal wastewater treatment. Chemosphere. 2014;113:36–41.
Repo E, Kurniawan TA, Warchol JK, Sillanpää MET. Removal of Co(II) and Ni(II) ions from contaminated water using silica gel functionalized with EDTA and/or DTPA as chelating agents. J Hazard Mater. 2009;171:1071–80.
Chai LY, Wang QW, Li QZ, Yang ZH, Wang YY. Enhanced removal of Hg(II) from acidic aqueous solution using thiol-functionalized biomass. Water Sci Technol. 2010;62:2157–65.
Wang QW, Qin WQ, Chai LY, Li QZ. Understanding the formation of colloidal mercury in acidic wastewater with high concentration of chloride ionsby electrocapillary curves. Environ Sci Pollut R. 2014;21:3866–72.
Özkahraman B, Özbaş Z, Öztürk AB. Synthesis of ion-imprinted alginate based beads: selective adsorption behavior of nickel (II) ions. J Polym Environ. 2018;26(11):4303–10.
Pourjavid MR, Arabieh M, Yousefi SR, Jamali MR, Rezaee M, Haji Hosseini M, et al. Study on column SPE with synthesized graphene oxide and FAAS for determination of trace amount of Co(II) and Ni(II) ions in real sample. Mater Sci Eng. 2015;47:114–22.
Rafati L, Nabizadeh R, Mahvi AH, Dehghani MH. Removal of phosphate from aqueous solutions by iron nano-particle resin Lewatit (FO36). Korean J Chem Eng. 2012;29(4):473–7.
Mauchauffée S, Meux E. Use of sodium decanoate for selective precipitation of metals contained in industrial wastewater. Chemosphere. 2007;69:763–8.
Rengaraj S, Yeon KH, Kang SY, Lee JU, Kim KW, Moon SH. Studies on adsorptive removal of co(II), Cr(III) and Ni(II) by IRN77 cation-exchange resin. J Hazard Mater. 2002;B92:185–98.
El Samrani AG, Lartiges BS, Villiéras F. Chemical coagulation of combined sewer overflow: heavy metal removal and treatment optimization. Water Res. 2008;42:951–60.
Rengaraj S, Moon SH. Kinetics of adsorption of Co(II) removal from water and wastewater by ion-exchange resins. Water Res. 2002;36:1783–93.
Mohsen-Nia M, Montazeri P, Modarress H. Removal of Cu2+and Ni2+from wastewater with a chelating agent and reverse osmosis processes. Desalination. 2007;217:276–81.
Kurniawan TA, Chan GYS, Lo WH, Babel S. Comparisons of low-cost adsor-bents for treating wastewaters laden with heavy metals. Sci Total Environ. 2006;366:409–26.
Gallego-Gallegos M, Muñoz-Olivas R, Martin-Esteban A, Cámara C. Synthesis and evaluation of molecularly imprinted polymers for organotin compounds: A screening method for tributyltin detection in seawater. Anal Chim Acta. 2005;531:33–9.
Ramstrom O, Ansell R. Molecular imprinting technology: challenges and prospects for the future. J Chirality. 1998;10:195–209.
Saraji M, Yousefi H. Selective solid-phase extraction of Ni(II) by an ion-imprinted polymer from water samples. J Hazard Mater. 2009;167:1152–7.
Rao TP, Kala R, Daniel S. Metal ion-imprinted polymers--novel materials for selective recognition of inorganics. Anal Chim Acta. 2001;578:105–16.
Burham N. Separation and preconcentration system for lead and cadmium determination in natural samples using 2-aminoacetylthiophenol modified polyurethane foam. Desalination. 2009;249:1199–205.
Rivera-Jiménez SM, Méndez-González S, Hernández-Maldonado A. Metal (M = Co2+, Ni2+, and Cu2+) grafted mesoporous SBA-15: effect of transition metal incorporation and pH conditions on the adsorption of naproxen from water. Microporous Mesoporous Mater. 2010;132:470–9.
Rafati L, Ehrampoush MH, Rafati AA, Mokhtari M, Mahvi AH. Nanocomposite adsorbent based on β-cyclodextrin-PVP-clay for the removal of naproxen from aqueous solution: fixed-bed column and modeling studies. Desalin Water Treat. 2018;132:63–74.
Zhang JN, Ma Z, Jiao J, Yin HF, Yan WF, Hagaman EW, et al. Layer-by-layer grafting of titanium phosphate onto Mesoporous silica SBA-15 surfaces: synthesis, characterization, and applications. Langmuir. 2009;25:12541–9.
Moradi Kalhor M, Rafati AA, Rafati L, Rafati AA. Synthesis, characterization and adsorption studies of amino functionalized silica nano hollow sphere as an efficient adsorbent for removal of imidacloprid pesticide. J Mol Liq. 2018;266:453–9.
Wang J, Zheng S, Shao Y, Liu J, Xu Z, Zhu D. Amino-functionalized Fe3O4@SiO2 core–shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal. J Colloid Interface Sci. 2010;349:293–9.
Wang S, Wang KA, Dai C, Shi H, Li J. Adsorption of Pb2+ on amino-functionalized core–shell magnetic mesoporous SBA-15 silica composite. Chem Eng J. 2015;262:897–903.
Pérez-Quintanilla D, del Hierro I, Fajardo M, Sierra I. Microporous Mesoporous Mater. 2006;89:58–68.
Tadjarodi A, Jalalat V, Zare-Dorabei R. Synthesis and characterization of functionalized SBA-15 Mesoporous Silica by N, N´-Bis(salicylidene)ethylenediamine Schiff-Base. J Nanotech Stract. 2013;3:477–82.
Faisal M, Ismail AA, Harraz FA, Bouzid H, Al-Sayari S, Al-Hajry A. Mesoporous TiO2 based optical sensor for highly sensitive and selective detection and preconcentration of Bi(III) ions. Chem Eng J. 2014;243:509–16.
Chmelka BF, Stucky GD, Zhao DY, Feng JL, Huo QS, Melosh N, et al. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Sci. 1998;279:548–52.
Jiang Y, Liu Y, Liu Z, Qiu J, Meng M, Ni L, et al. Selective Ce(III) ion-imprinted polymer grafted on Fe3O4 nanoparticles supported by SBA-15 mesopores microreactor via surface-initiated RAFT polymerization. Microporous Mesoporous Mater. 2016;234:176–85.
Kang YS, Zhao S, Lee DK, Kim Ch W, Cha HG, Kim YH. Synthesis of magnetic nanoparticles of Fe3O4 and CoFe2O4 and their surface modification by surfactant adsorption. Bull Korean Chem Soc. 2006;27:237–42.
Kazeminezhad I, Mosivand S. Phase transition of Electrooxidized Fe3O4 to γ and α-Fe2O3 nanoparticles using sintering treatment. Acta Phys Pol. 2014;A125:1210–4.
Bagheri A, Behbahani M, Taghizadeh M, Salarian M, Sadeghi O, Adlnasab L, et al. Synthesis and characterization of nano structure lead (II) ion-imprinted polymer as a new sorbent for selective extraction and preconcentration of ultra trace amounts of lead ions from vegetables, rice, and fish samples. Food Chem. 2013;138(2–3):2050–6.
Sayar O, Akbarzadeh Torbati N, Saravani H, Mehrani K, Behbahani A, Moghadam ZH. A novel magnetic ion imprinted polymer for selective adsorption of trace amounts of lead(II) ions in environment samples. J Ind Eng Chem. 2014;20(5):2657–62.
Fayazi M, Taher MA, Afzali D, Mostafavi A, Ghanei-Motlagh M. Synthesis and application of novel ion-imprinted polymer coated magnetic multi-walled carbon nanotubes for selective solid phase extraction of lead(II) ions. Mater Sci Eng. 2016;C(60):365–73.
Zhang H, Dou Q, Jin X, Sun D, Wang D, Yang T. Magnetic Pb(II) Ion-imprinted polymer prepared by surface imprinting technique and its adsorption properties. Sep Sci Technol. 2015;50:901–10.
Xu X, Wang M, Wu Q, Xu Z, Tian X. Synthesis and application of novel magnetic ion-imprinted polymers for selective solid phase extraction of Cadmium (II). Polym. 2017;(9):360–70.
Xi Y, Luo Y, Luo J, Luo X. Removal of Cadmium(II) from wastewater using novel cadmium ion-imprinted polymers. J Chem Eng Data. 2015;60(11):3253–326.
Luo X, Luo S, Zhan Y, Shu H, Huang Y, Tu X. Novel Cu (II) magnetic ion imprinted materials prepared by surface imprinted technique combined with a sol–gel process. J Hazard Mater. 2011;192:949–55.
Kang R, Qiu L, Fang L, Yu R, Chen Y, Lu X, et al. A novel magnetic and hydrophilic ion-imprinted polymer as a selective sorbent for the removal of cobalt ions from industrial wastewater. J Environ Chem Eng. 2016;(4):2268–77.
Yan L, Zhancha L, Jiangdong D, Jie G, Jimin X, Yongsheng Y. Selective adsorption of Co(II) by Mesoporous Silica SBA-15-supported surface ion imprinted polymer: kinetics, isotherms, and thermodynamics studies. Chin J Chem. 2011;29:387–98.
Khoddami N, Shemirani F. A new magnetic ion-imprinted polymer as a highly selective sorbent for determination of cobalt in biological and environmental samples. Talanta. 2016;146:244–52.
Rafati L, Ehrampoush MH, Rafati AA, Mokhtari M, Mahvi AH. Removal of ibuprofen from aqueous solution by functionalized strong nano-clay composite adsorbent: kinetic and equilibrium isotherm studies. Int J Environ Sci Technol. 2018;15:513–24.
Langmuir. The constitution and fundamental properties of solids and liquids. J Am Chem Soc. 1916;38(5):2221–95.
Freundlich HMF. Over the adsorption in solution. Z Phys Chem. 1906;57A(5):385–470.
Redlich O, Peterson DL. A useful adsorption isotherm. J Phys Chem. 1959;63(5):1024.
Juang RS, Wu FC, Tseng RL. Adsorption isotherms of phenolic compounds from aqueous solutions onto activated carbon fibers. J Chem Eng Data. 1996;41(3):487–92.
Yang RT. Adsorbents: fundamentals and applications. New Jersey: Wiley; 2003.
Allen SJ, Mckay G, Porter JF. Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems. J Colloid Interface Sci. 2004;280(2):322–33.
Foo KY, Hameed BH. Insights into the modeling of adsorption isotherm systems. Chem Eng J. 2010;156(1):2–10.
Rafati L, Ehrampoush MH, Rafati AA, Mokhtari M, Mahvi AH. Modeling of adsorption kinetic and equilibrium isotherms of naproxen onto functionalized nano-clay composite adsorbent. J Mol Liq. 2016;224:832–41.
Kamranifar M, Khodadadi M, Samiei V, Dehdashti B, Noori Sepehr M, Rafati L, et al. Comparison the removal of reactive red 195 dye using powder and ash of barberry stem as a low cost adsorbent from aqueous solutions: isotherm and kinetic study. J Mol Liq. 2018;255:572–7.
Gunay A, Arslankaya E, Tosun I. Lead removal from aqueous solution by natural and pretreated clinoptilolite: Adsorption equilibrium and kinetics. J Hazard Mater. 2007;146:362–71.
Martins RJE, Vilar VJP, Boaventura RAR. Kinetic modelling of cadmium and lead removal by aquatic mosses. Braz J Chem Eng. 2014;31(1):229–42.
Bektas N, Agım BA, Kara S. Kinetic and equilibrium studies in removing lead ions from aqueous solutions by natural sepiolite. J Hazard Mater. 2004;B(112):115–22.
Fan T, Liu Y, Feng B, Zeng G, Yang C, Zhou M, et al. Biosorption of cadmium(II), zinc(II) and lead(II) by Penicillium simplicissimum: Isotherms, kinetics and thermodynamics. J Hazard Mater. 2008;160:655–61.
Huang Ch HB. Silica-coated magnetic nanoparticles modified with γ- mercaptopropyltrimethoxysilane for fast and selective solid phase extraction of trace amounts of Cd, Cu, Hg, and Pb in environmental and biological samples prior to their determination by inductively coupled plasma mass spectrometry. Spectrochim. Acta. 2008;63:437–44.
Nassar MY, Ahmed IS, Mohamed TY, Khatab M. A controlled, templatefree, and hydrothermal synthesis route to sphere-like [small alpha]-Fe2O3 nanostructures for textile dye removal. RSC Adv. 2016;6:20001–13.
Aksakal O, Ucun H. Equilibrium, kinetic and thermodynamic studies of the biosorption of textile dye (reactive red 195) onto Pinus sylvestris L. J Hazard Mater. 2010;181:666–72.
Xue A, Zhou S, Zhao Y, Lu X, Han P. Adsorption of reactive dyes from aqueous solution by silylated palygorskite. Appl Clay Sci. 2010;48:638–40.
Dursun AY, Tepe O. Removal of Chemazol reactive red 195 from aqueous solution by dehydrated beet pulp carbon. J Hazard Mater. 2011;194:303–11.
Ibezim-Ezeani MU, Okoye FA, Akaranta O. Studies on the ion exchange properties of modified and unmodified orange mesocarp extract in aqueous solution. Int Arch Appl Sci Technol. 2010;1:33–40.
Ramila A, Munoz B, Pariente JP, Regí MV. Mesoporous MCM-41 as drug host system. J Sole Gel Sci Technol. 2003;26:1199–202.
Andersson J, Rosenholm J, Areva S, Linden M. Influences of material char- acteristics on ibuprofen drug loading and release profiles from ordered micro-and mesoporous silica matrices. Chem Mater. 2004;16:4160–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declaration on conflict of interest
There is no conflict of Interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 363 kb)
Rights and permissions
About this article
Cite this article
Adibmehr, Z., Faghihian, H. Preparation of highly selective magnetic cobalt ion-imprinted polymer based on functionalized SBA-15 for removal Co2+ from aqueous solutions. J Environ Health Sci Engineer 17, 1213–1225 (2019). https://doi.org/10.1007/s40201-019-00439-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40201-019-00439-x