Abstract
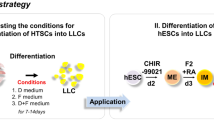
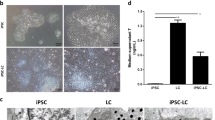
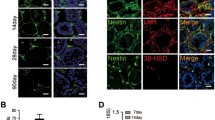
95% of the body’s testosterone is produced by the Leydig Cells (LCs) in adult testis, and LC functional degradation can cause testosterone deficiency ultimately leading towards hypogonadism. The transplantation of LCs derived from stem cells is a very promising therapy to overcome the testosterone deficiency. The isolated umbilical cord mesenchymal stem cells (UMSCs) were identified by flow cytometry and adipogenic and osteogenic differentiation. Western blotting and reverse transcription polymerase chain reaction (RT-PCR) were used for the differentiated Leydig-like cell identification. The comparisons of the testosterone levels, gene expression levels, and cyclic adenosine monophosphate (cAMP) productions were performed through radioimmunoassay, quantitative polymerase chain reaction (qPCR), and cAMP assay kit, respectively. Here, it is stated that our isolated human UMSCs, which could positively express CD29, CD44, CD59, CD90, CD105, and CD166 but negatively express CD34 as well as could be differentiated into adipocytes and osteocytes, could be differentiated into Leydig-like cells (UMSC-LCs) using a novel differentiation method based on molecular compounds. The enrichment UMSC-LCs could secrete testosterone into the medium supernatant and produce considerable cAMP at the stimulation of luteinizing hormone (LH), and positively expressed LC lineage-typical markers LHCGR, SCARB1, SATR, CYP11A1, CYP17A1, HSD3B1, HSD17B3, and SF-1 as well as negatively expressed mesenchymal stem cell typical markers CD29, CD44, and CD105. The expression levels of NR3C4, PDGFRA, and NR3A1 in UMSC-LCs were higher than those of UMSCs and were comparable with LCs. These results illuminated that UMSCs could be differentiated into Leydig-like cells using the defined molecular compounds, which might further support MSC-derived Leydig cell transplantation therapy for testosterone insufficiency.




Similar content being viewed by others
References
Wohlfahrt-Veje C, Main KM, Skakkebaek NE. Testicular dysgenesis syndrome: foetal origin of adult reproductive problems. Clin Endocrinol. 2009;71:459–65.
Nef S, Parada LF. Hormones in male sexual development. Genes Dev. 2000;14:3075–86.
Zirkin B. Where do adult Leydig cells come from? Biol Reprod. 2010;82:1019–20.
Smith LB, Walker WH. The regulation of spermatogenesis by androgens. Semin Cell Dev Biol. 2014;30:2–13.
Basaria S. Male hypogonadism. Lancet. 2014;383:1250–63.
Huhtaniemi I. Late-onset hypogonadism: current concepts and controversies of pathogenesis, diagnosis and treatment. Asian J Androl. 2014;16:192–202.
McHenry MC. Testosterone deficiency in older men: a problem worth treating. Consult Pharm. 2012;27:152–63.
Page ST, Amory JK, Bowman FD, et al. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2005;90:1502–10.
Seftel AD. Re: critical update of the 2010 endocrine society clinical practice guidelines for male hypogonadism: a systematic analysis. J Urol. 2016;195:441.
Klotz L. Testosterone therapy and prostate cancer—safety concerns are well founded. Nat Rev Urol. 2015;12:48–544.
Yeap B. Testosterone and cardiovascular disease risk. Curr Opin Endocrinol Diabetes Obes. 2015;22:193–202.
Machluf M, Orsola A, Boorjian S, Kershen R, Atala A. Microencapsulation of Leydig cells: a system for testosterone supplementation. Endocrinology. 2003;144:4975–9.
Svechnikov K, Landreh L, Weisser J, et al. Origin, development and regulation of human Leydig cells. Horm Res Paediatr. 2010;73:93–101.
Mobasheri A, Csaki C, Clutterbuck AL, Rahmanzadeh M, Shakibaei M. Mesenchymal stem cells in connective tissue engineering and regenerative medicine: applications in cartilage repair and osteoarthritis therapy. Histol Histopathol. 2009;24:347–66.
Gondo S, Okabe T, Tanaka T, et al. Adipose tissue-derived and bone marrow-derived mesenchymal cells develop into different lineage of steroidogenic cells by forced expression of steroidogenic factor 1. Endocrinology. 2008;149:4717–25.
Yazawa T, Mizutani T, Yamada K, et al. Differentiation of adult stem cells derived from bone marrow stroma into Leydig or adrenocortical cells. Endocrinology. 2006;147:4104–11.
Xing X, Zhang Z, Zhong L, et al. Differentiation of human umbilical cord mesenchymal stem cells into steroidogenic cells in vitro. Exp Ther Med. 2016;12:3527–34.
Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301–16.
Zhao Q, Hu J, Xiang J, et al. Intranasal administration of human umbilical cord mesenchymal stem cells-conditioned medium enhances vascular remodeling after stroke. Brain Res. 2015;1624:489–96.
Guo X, Li S, Ji Q, Lian R, Chen J. Enhanced viability and neural differential potential in poor post-thaw hADSCs by agarose multi-well dishes and spheroid culture. Hum Cell. 2015;28:175–89.
Wei X, Peng G, Zheng S, Wu X. Differentiation of umbilical cord mesenchymal stem cells into steroidogenic cells in comparison to bone marrow mesenchymal stem cells. Cell Prolif. 2012;45:101–10.
Ge R, Hardy M. Decreased cyclin A2 and increased cyclin G1 levels coincide with loss of proliferative capacity in rat Leydig cells during pubertal development. Endocrinology. 1997;138:3719–26.
Ge R, Hardy M. Variation in the end products of androgen biosynthesis and metabolism during postnatal differentiation of rat Leydig cells. Endocrinology. 1998;139:3787–95.
Payne A, Wong K, Vega M. Differential effects of single and repeated administrations of gonadotropins on luteinizing hormone receptors and testosterone synthesis in two populations of Leydig cells. J Biol Chem. 1980;255:7118–222.
Li X, Wang Z, Jiang Z, et al. Regulation of seminiferous tubule-associated stem Leydig cells in adult rat testes. Proc Natl Acad Sci USA. 2016;113:2666–711.
Mitchell R, Childs A, Anderson R, et al. Do phthalates affect steroidogenesis by the human fetal testis? Exposure of human fetal testis xenografts to di-n-butyl phthalate. J Clin Endocrinol Metab. 2012;97:E341–E348348.
Chen H, Guo J, Ge R, Lian Q, Papadopoulos V, Zirkin BR. Steroidogenic fate of the Leydig cells that repopulate the testes of young and aged Brown Norway rats after elimination of the preexisting Leydig cells. Exp Gerontol. 2015;72:8–15.
Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7.
Bojesen A, Juul S, Gravholt C. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J Clin Endocrinol Metab. 2003;88:622–6.
Hagenäs L. Normal and deviating puberty in boys. Tidsskr Nor Laegeforen. 2008;128:1284–8.
Rogol A. Pubertal androgen therapy in boys. Pediatr Endocrinol Rev. 2005;2:383–90.
Sun J, Xi Y, Zhang Z, et al. Leydig cell transplantation restores androgen production in surgically castrated prepubertal rats. Asian J Androl. 2009;11:405–9.
Dong Y, Zhang Q, Li Y, Jiang J, Chen S. Enhancement of tendon-bone healing for anterior cruciate ligament (ACL) reconstruction using bone marrow-derived mesenchymal stem cells infected with BMP-2. Int J Mol Sci. 2012;13:13605–20.
Tsai C, Huang T, Ma H, Chiang E, Hung S. Isolation of mesenchymal stem cells from shoulder rotator cuff: a potential source for muscle and tendon repair. Cell Transplant. 2013;22:413–22.
Liu S, Shao Y, Lin Q, Liu H, Zhang D. 7,8-Dihydroxy coumarin promotes chondrogenic differentiation of adipose-derived mesenchymal stem cells. J Int Med Res. 2013;41:82–96.
Li X, Bai J, Ji X, Li R, Xuan Y, Wang Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int J Mol Med. 2014;34:695–704.
Jadhav U, Jameson JL. Steroidogenic factor-1 (SF-1)-driven differentiation of murine embryonic stem (ES) cells into a gonadal lineage. Endocrinology. 2011;152:2870–82.
Sonoyama T, Sone M, Honda K, et al. Differentiation of human embryonic stem cells and human induced pluripotent stem cells into steroid-producing cells. Endocrinology. 2012;153:4336–455.
Vassena R, Eguizabal C, Heindryckx B, et al. Stem cells in reproductive medicine: ready for the patient? Hum Reprod. 2015;30:2014–21.
Chen H, Ge R, Zirkin B. Leydig cells: From stem cells to aging. Mol Cell Endocrinol. 2009;306:9–16.
Chen H, Stanley E, Jin S, Zirkin B. Stem Leydig cells: from fetal to aged animals. Birth Defects Res C Embryo Today. 2010;90:272–83.
Haider S. Cell biology of Leydig cells in the testis. Int Rev Cytol. 2004;233:181–241.
Mendis-Handagama S, Ariyaratne H. Differentiation of the adult Leydig cell population in the postnatal testis. Biol Reprod. 2001;65:660–71.
Ye L, Li X, Li L, Chen H, Ge R. Insights into the development of the adult Leydig cell lineage from stem Leydig cells. Front Physiol. 2017;8:430.
Kawai Y, Noguchi J, Akiyama K, et al. A missense mutation of the Dhh gene is associated with male pseudohermaphroditic rats showing impaired Leydig cell development. Reproduction. 2011;141:217–25.
Pagotto M, Roldán M, Pagotto R, et al. Localization and functional activity of cytochrome P450 side chain cleavage enzyme (CYP11A1) in the adult rat kidney. Mol Cell Endocrinol. 2011;332:253–60.
Hammer G, Parker K, Schimmer B. Minireview: transcriptional regulation of adrenocortical development. Endocrinology. 2005;146:1018–24.
Luo X, Ikeda Y, Parker K. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–90.
Odeh H, Kleinguetl C, Ge R, Zirkin B, Chen H. Regulation of the proliferation and differentiation of Leydig stem cells in the adult testis. Biol Reprod. 2014;90:123.
Murono E, Washburn A, Goforth D, Wu N. Basic fibroblast growth factor-induced increase in 125I-human chorionic gonadotropin binding to luteinizing hormone receptors in cultured immature Leydig cells is mediated by binding to heparan sulfate proteoglycans. Mol Cell Endocrinol. 1993;97:109–14.
Shan L, Zhu L, Bardin C, Hardy M. Quantitative analysis of androgen receptor messenger ribonucleic acid in developing Leydig cells and Sertoli cells by in situ hybridization. Endocrinology. 1995;136:3856–62.
Kilcoyne K, Smith L, Atanassova N, et al. Fetal programming of adult Leydig cell function by androgenic effects on stem/progenitor cells. Proc Natl Acad Sci USA. 2014;111:E1924–E19321932.
Dombrowicz D, Sente B, Reiter E, Closset J, Hennen G. Pituitary control of proliferation and differentiation of Leydig cells and their putative precursors in immature hypophysectomized rat testis. J Androl. 1996;17:639–50.
Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72.
Stocco DM. StAR protein and the regulation of steroid hormone biosynthesis. Annu Rev Physiol. 2001;63:193–21313.
Acknowledgements
We thank the grants supported by the National Nature Science Foundation of China (81701426 and 81970435), the Natural Science Foundation of Zhejiang Province (LY20H040003 and LY18H160058), the Medical and Health Research Science and Technology Plan Project of Zhejiang Province (2018KY523), and the Public Welfare Science and Technology Plan Project of Wenzhou City (Y20180097 and Y20190162).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in study involving human participants were in accordance with the ethical standards of Human Research and Ethical Committee of Wenzhou Medical University as well as the Helsinki Declaration.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ji, W., Chen, Y., Wang, L. et al. Differentiation of human umbilical cord mesenchymal stem cells into Leydig-like cells with defined molecular compounds. Human Cell 33, 318–329 (2020). https://doi.org/10.1007/s13577-020-00324-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-020-00324-y