Abstract
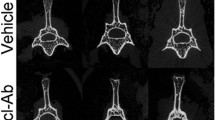
In osteogenesis imperfecta (OI), vertebrae brittleness causes thorax deformations and leads to cardiopulmonary failure. As sclerostin-neutralizing antibodies increase bone mass and strength in animal models of osteoporosis, their administration in two murine models of severe OI enhanced the strength of vertebrae in growing female Crtap−/− mice but not in growing male Col1a1Jrt/+ mice. However, these two studies ignored the impact of antibodies on spine growth, fracture rates, and compressive mechanical properties. Here, we conducted a randomized controlled trial in oim/oim mice, an established model of human severe OI type III due to a mutation in Col1a2. Five-week-old female WT and oim/oim mice received either PBS or sclerostin antibody (Scl-Ab) for 9 weeks. Analyses included radiography, histomorphometry, pQCT, microcomputed tomography, and biomechanical testing. Though it did not modify vertebral axial growth, Scl-Ab treatment markedly reduced the fracture prevalence in the pelvis and caudal vertebrae, enhanced osteoblast activity (L4), increased cervico-sacral spine BMD, and improved the lumbosacral spine bone cross-sectional area. Scl-Ab did not impact vertebral height and body size but enhanced the cortical thickness and trabecular bone volume significantly in the two Scl-Ab groups. At lumbar vertebrae and tibial metaphysis, the absolute increase in cortical and trabecular bone mass was higher in Scl-Ab WT than in Scl-Ab oim/oim. The effects on trabecular bone mass were mainly due to changes in trabecular number at vertebrae and in trabecular thickness at metaphyses. Additionally, Scl-Ab did not restore a standard trabecular network, but improved bone compressive ultimate load with more robust effects at vertebrae than at metaphysis. Overall, Scl-Ab treatment may be beneficial for reducing vertebral fractures and spine deformities in patients with severe OI.




Similar content being viewed by others
References
Rauch F, Glorieux FH (2004) Osteogenesis imperfecta. The Lancet 363(9418):1377–1385
Forlino A, Cabral WA, Barnes AM, Marini JC (2011) New perspectives on osteogenesis imperfecta. Nat Rev Endocrinol 7(9):540–557
Van Dijk FS, Sillence DO (2014) Osteogenesis imperfecta: clinical diagnosis, nomenclature and severity assessment. Am J Med Genet A 164A(6):1470–1481
LoMauro A, Fraschini P, Pochintesta S, Romei M, D'Angelo MG, Aliverti A (2018) Ribcage deformity and the altered breathing pattern in children with osteogenesis imperfecta. Pediatr Pulmonol 53(7):964–972
McAllion SJ, Paterson CR (1996) Causes of death in osteogenesis imperfecta. J Clin Pathol 49(8):627–630
Franzone JM, Shah SA, Wallace MJ, Kruse RW (2019) Osteogenesis imperfecta: a pediatric orthopedic perspective. Orthop Clin North Am 50(2):193–209
Cheung MS, Glorieux FH (2008) Osteogenesis Imperfecta: update on presentation and management. Rev Endocr Metab Disord 9(2):153–160
Dwan K, Phillipi CA, Steiner RD, Basel D (2016) Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database Syst Rev 10:CD005088
Tashjian AH Jr, Goltzman D (2008) On the interpretation of rat carcinogenicity studies for human PTH(1–34) and human PTH(1–84). J Bone Miner Res 23(6):803–811
Chouinard L, Felx M, Mellal N, Varela A, Mann P, Jolette J, Samadfam R, Smith SY, Locher K, Buntich S, Ominsky MS, Pyrah I, Boyce RW (2016) Carcinogenicity risk assessment of romosozumab: a review of scientific weight-of-evidence and findings in a rat lifetime pharmacology study. Regul Toxicol Pharmacol 81:212–222
Ke HZ, Richards WG, Li X, Ominsky MS (2012) Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev 33(5):747–783
Baron R, Kneissel M (2013) WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19(2):179–192
McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, Langdahl BL, Reginster JY, Zanchetta JR, Wasserman SM, Katz L, Maddox J, Yang YC, Libanati C, Bone HG (2014) Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 370(5):412–420
Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, Hofbauer LC, Lau E, Lewiecki EM, Miyauchi A, Zerbini CA, Milmont CE, Chen L, Maddox J, Meisner PD, Libanati C, Grauer A (2016) Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med 375(16):1532–1543
Roschger A, Roschger P, Keplingter P, Klaushofer K, Abdullah S, Kneissel M, Rauch F (2014) Effect of sclerostin antibody treatment in a mouse model of severe osteogenesis imperfecta. Bone 66:182–188
Sinder BP, Eddy MM, Ominsky MS, Caird MS, Marini JC, Kozloff KM (2013) Sclerostin antibody improves skeletal parameters in a Brtl/+ mouse model of osteogenesis imperfecta. J Bone Miner Res 28(1):73–80
Sinder BP, White LE, Salemi JD, Ominsky MS, Caird MS, Marini JC, Kozloff KM (2014) Adult Brtl/+ mouse model of osteogenesis imperfecta demonstrates anabolic response to sclerostin antibody treatment with increased bone mass and strength. Osteoporos Int 25(8):2097–2107
Sinder BP, Salemi JD, Ominsky MS, Caird MS, Marini JC, Kozloff KM (2015) Rapidly growing Brtl/+ mouse model of osteogenesis imperfecta improves bone mass and strength with sclerostin antibody treatment. Bone 71:115–123
Jacobsen CM, Barber LA, Ayturk UM, Roberts HJ, Deal LE, Schwartz MA, Weis M, Eyre D, Zurakowski D, Robling AG, Warman ML (2014) Targeting the LRP5 pathway improves bone properties in a mouse model of osteogenesis imperfecta. J Bone Miner Res 29(10):2297–2306
Grafe I, Alexander S, Yang T, Lietman C, Homan EP, Munivez E, Chen Y, Jiang MM, Bertin T, Dawson B, Asuncion F, Ke HZ, Ominsky MS, Lee B (2016) Sclerostin antibody treatment improves the bone phenotype of Crtap(-/-) mice, a model of recessive osteogenesis imperfecta. J Bone Miner Res 31(5):1030–1040
Joeng KS, Lee YC, Jiang MM, Bertin TK, Chen Y, Abraham AM, Ding H, Bi X, Ambrose CG, Lee BH (2014) The swaying mouse as a model of osteogenesis imperfecta caused by WNT1 mutations. Hum Mol Genet 23(15):4035–4042
Joeng KS, Lee YC, Lim J, Chen Y, Jiang MM, Munivez E, Ambrose C, Lee BH (2017) Osteocyte-specific WNT1 regulates osteoblast function during bone homeostasis. J Clin Invest 127(7):2678–2688
Chipman SD, Sweet HO, McBride DJ Jr, Davisson MT, Marks SC Jr, Shuldiner AR, Wenstrup RJ, Rowe DW, Shapiro JR (1993) Defective pro alpha 2(I) collagen synthesis in a recessive mutation in mice: a model of human osteogenesis imperfecta. Proc Natl Acad Sci USA 90(5):1701–1705
Carleton SM, McBride DJ, Carson WL, Huntington CE, Twenter KL, Rolwes KM, Winkelmann CT, Morris JS, Taylor JF, Phillips CL (2008) Role of genetic background in determining phenotypic severity throughout postnatal development and at peak bone mass in Col1a2 deficient mice (oim). Bone 42(4):681–694
Camacho NP, Raggio CL, Doty SB, Root L, Zraick V, Ilg WA, Toledano TR, Boskey AL (2001) A controlled study of the effects of alendronate in a growing mouse model of osteogenesis imperfecta. Calcif Tissue Int 69(2):94–101
Misof BM, Roschger P, Baldini T, Raggio CL, Zraick V, Root L, Boskey AL, Klaushofer K, Fratzl P, Camacho NP (2005) Differential effects of alendronate treatment on bone from growing osteogenesis imperfecta and wild-type mouse. Bone 36(1):150–158
Mehrotra M, Rosol M, Ogawa M, Larue AC (2010) Amelioration of a mouse model of osteogenesis imperfecta with hematopoietic stem cell transplantation: microcomputed tomography studies. Exp Hematol 38(7):593–602
Vanleene M, Saldanha Z, Cloyd KL, Jell G, Bou-Gharios G, Bassett JH, Williams GR, Fisk NM, Oyen ML, Stevens MM, Guillot PV, Shefelbine SJ (2011) Transplantation of human fetal blood stem cells in the osteogenesis imperfecta mouse leads to improvement in multiscale tissue properties. Blood 117(3):1053–1060
Cardinal M, Tys J, Roels T, Lafont S, Ominsky MS, Devogelaer JP, Chappard D, Mabilleau G, Ammann P, Nyssen-Behets C, Manicourt DH (2019) Sclerostin antibody reduces long bone fractures in the oim/oim model of osteogenesis imperfecta. Bone 124:137–147
Yao X, Carleton SM, Kettle AD, Melander J, Phillips CL, Wang Y (2013) Gender-dependence of bone structure and properties in adult osteogenesis imperfecta murine model. Ann Biomed Eng 41(6):1139–1149
Cesarovic N, Nicholls F, Rettich A, Kronen P, Hassig M, Jirkof P, Arras M (2010) Isoflurane and sevoflurane provide equally effective anaesthesia in laboratory mice. Lab Anim 44(4):329–336
Cornelis MA, Mahy P, Devogelaer JP, De Clerck HJ, Nyssen-Behets C (2010) Does orthodontic loading influence bone mineral density around titanium miniplates? An experimental study in dogs. Orthod Craniofac Res 13(1):21–27
Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 28(1):2–17
Boivin G, Meunier PJ (2002) The degree of mineralization of bone tissue measured by computerized quantitative contact microradiography. Calcif Tissue Int 70(6):503–511
Ammann P, Badoud I, Barraud S, Dayer R, Rizzoli R (2007) Strontium ranelate treatment improves trabecular and cortical intrinsic bone tissue quality, a determinant of bone strength. J Bone Miner Res 22(9):1419–1425
Smith L, Bigelow EM, Jepsen KJ (2013) Systematic evaluation of skeletal mechanical function. Curr Protoc Mouse Biol 3:39–67
Ammann P, Bonjour JP, Rizzoli R (2000) Essential amino acid supplements increase muscle weight, bone mass and bone strength in adult osteoporotic rats. J Musculoskelet Neuronal Interact 1(1):43–44
Pihlajaniemi T, Dickson LA, Pope FM, Korhonen VR, Nicholls A, Prockop DJ, Myers JC (1984) Osteogenesis imperfecta: cloning of a pro-alpha 2(I) collagen gene with a frameshift mutation. J Biol Chem 259(21):12941–12944
Camacho NP, Hou L, Toledano TR, Ilg WA, Brayton CF, Raggio CL, Root L, Boskey AL (1999) The material basis for reduced mechanical properties in oim mice bones. J Bone Miner Res 14(2):264–272
Kalajzic I, Terzic J, Rumboldt Z, Mack K, Naprta A, Ledgard F, Gronowicz G, Clark SH, Rowe DW (2002) Osteoblastic response to the defective matrix in the osteogenesis imperfecta murine (oim) mouse. Endocrinology 143(5):1594–1601
Miller E, Delos D, Baldini T, Wright TM, Pleshko Camacho N (2007) Abnormal mineral-matrix interactions are a significant contributor to fragility in oim/oim bone. Calcif Tissue Int 81(3):206–214
Rowe D (2008) Osteogenesis imperfecta in principles of bone biology. In: Bilezikian JP, Raisz LG, Martin TJ (eds) 3rd edn. Elsevier, Amsterdam, pp 1511–1531
McCarthy EA, Raggio CL, Hossack MD, Miller EA, Jain S, Boskey AL, Camacho NP (2002) Alendronate treatment for infants with osteogenesis imperfecta: demonstration of efficacy in a mouse model. Pediatr Res 52(5):660–670
Bargman R, Huang A, Boskey AL, Raggio C, Pleshko N (2010) RANKL inhibition improves bone properties in a mouse model of osteogenesis imperfecta. Connect Tissue Res 51(2):123–131
Bargman R, Posham R, Boskey AL, DiCarlo E, Raggio C, Pleshko N (2012) Comparable outcomes in fracture reduction and bone properties with RANKL inhibition and alendronate treatment in a mouse model of osteogenesis imperfecta. Osteoporos Int 23(3):1141–1150
Berman AG, Wallace JM, Bart ZR, Allen MR (2016) Raloxifene reduces skeletal fractures in an animal model of osteogenesis imperfecta. Matrix Biol 52–54:19–28
Shi C, Hu B, Guo L, Cao P, Tian Y, Ma J, Chen Y, Wu H, Hu J, Deng L, Zhang Y, Yuan W (2016) Strontium ranelate reduces the fracture incidence in a growing mouse model of osteogenesis imperfecta. J Bone Miner Res 31(5):1003–1014
Smit TH (2002) The use of a quadruped as an in vivo model for the study of the spine—biomechanical considerations. Eur Spine J 11(2):137–144
Schendel MJ, Dekutoski MB, Ogilvie JW, Olsewski JM, Wallace LJ (1995) Kinematics of the canine lumbar intervertebral joint. An in vivo study before and after adjacent instrumentation. Spine 20(23):2555–2564
Carriero A, Zimmermann EA, Paluszny A, Tang SY, Bale H, Busse B, Alliston T, Kazakia G, Ritchie RO, Shefelbine SJ (2014) How tough is brittle bone? Investigating osteogenesis imperfecta in mouse bone. J Bone Miner Res 29(6):1392–1401
Bouxsein M (2013) Overview of bone structure and strength. In: Thakker R, Whyte MP, Eisman JA, Igarashi T (eds) Genetics of bone biology and skeletal disease. Elsevier Academic Press, Amsterdam, pp 25–34
Legrand E, Chappard D, Pascaretti C, Duquenne M, Krebs S, Rohmer V, Basle MF, Audran M (2000) Trabecular bone microarchitecture, bone mineral density, and vertebral fractures in male osteoporosis. J Bone Miner Res 15(1):13–19
Wegrzyn J, Roux JP, Arlot ME, Boutroy S, Vilayphiou N, Guyen O, Delmas PD, Chapurlat R, Bouxsein ML (2010) Role of trabecular microarchitecture and its heterogeneity parameters in the mechanical behavior of ex vivo human L3 vertebrae. J Bone Miner Res 25(11):2324–2331
Genant HK, Delmas PD, Chen P, Jiang Y, Eriksen EF, Dalsky GP, Marcus R, San Martin J (2007) Severity of vertebral fracture reflects deterioration of bone microarchitecture. Osteoporos Int 18(1):69–76
Land C, Rauch F, Munns CF, Sahebjam S, Glorieux FH (2006) Vertebral morphometry in children and adolescents with osteogenesis imperfecta: effect of intravenous pamidronate treatment. Bone 39(4):901–906
Christiansen BA, Kopperdahl DL, Kiel DP, Keaveny TM, Bouxsein ML (2011) Mechanical contributions of the cortical and trabecular compartments contribute to differences in age-related changes in vertebral body strength in men and women assessed by QCT-based finite element analysis. J Bone Miner Res 26(5):974–983
Roux JP, Wegrzyn J, Arlot ME, Guyen O, Delmas PD, Chapurlat R, Bouxsein ML (2010) Contribution of trabecular and cortical components to biomechanical behavior of human vertebrae: an ex vivo study. J Bone Miner Res 25(2):356–361
Silva MJ, Gibson LJ (1997) Modeling the mechanical behavior of vertebral trabecular bone: effects of age-related changes in microstructure. Bone 21(2):191–199
Zimmerman SM, Heard-Lipsmeyer ME, Dimori M, Thostenson JD, Mannen EM, O'Brien CA, Morello R (2018) Loss of RANKL in osteocytes dramatically increases cancellous bone mass in the osteogenesis imperfecta mouse (oim). Bone Rep 9:61–73
Holdsworth G, Greenslade K, Jose J, Stencel Z, Kirby H, Moore A, Ke HZ, Robinson MK (2018) Dampening of the bone formation response following repeat dosing with sclerostin antibody in mice is associated with up-regulation of Wnt antagonists. Bone 107:93–103
Ominsky MS, Niu QT, Li C, Li X, Ke HZ (2014) Tissue-level mechanisms responsible for the increase in bone formation and bone volume by sclerostin antibody. J Bone Miner Res. 29(6):1424–1430
Recker RR, Benson CT, Matsumoto T, Bolognese MA, Robins DA, Alam J, Chiang AY, Hu L, Krege JH, Sowa H, Mitlak BH, Myers SL (2015) A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density. J Bone Miner Res 30(2):216–224
Acknowledgements
The authors thank the Amgen-UCB consortium for providing the anti-sclerostin antibody (DHM). They are grateful to Isabelle Badoud, Jennifer Siebenaler and Walter Hudders for their expertise and assistance.
Funding
This research is supported by the Medical Scientific Research Fund (FRSM-IREC88A6, DHM) and by the French-speaking Belgian Association of the Osteogenesis Imperfecta (www.fetealavie.be, www.afboi.be/).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Michael S. Ominsky is a former employee and stockholder of Amgen. Mickaël Cardinal, Alicia Dessain, Thomas Roels, Sébastien Lafont, Jean-Pierre Devogelaer, Daniel Chappard, Guillaume Mabilleau, Patrick Ammann, Catherine Nyssen-Behets and Daniel H. Manicourt declare that they have no other conflict of interest.
Human and Animal Rights and Informed Consent
This study was approved by the Ethics Committee for Animal Care of the Université Catholique de Louvain under the reference number: 2014/UCL/MD/021, and conformed to the Belgian federal law for animal care.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cardinal, M., Dessain, A., Roels, T. et al. Sclerostin-Antibody Treatment Decreases Fracture Rates in Axial Skeleton and Improves the Skeletal Phenotype in Growing oim/oim Mice. Calcif Tissue Int 106, 494–508 (2020). https://doi.org/10.1007/s00223-019-00655-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-019-00655-5