Abstract
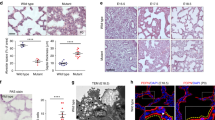
Pulmonary fibrosis is a severely disabling disease often leading to death. CCN2 (Cellular Communication Network factor 2, also known as CTGF) is a known mediator of fibrosis and clinical trials studying anti-CCN2 efficacy in pulmonary fibrosis are currently underway. Fork head box D1 (FoxD1) transcription factor is transiently expressed in several mesenchymal cell types, including those of fetal lungs. Differentiation of FoxD1-progenitor derived pericytes into myofibroblasts involves CCN2 expression and contributes importantly to maladaptive tissue remodeling in e.g. kidney and lung fibrosis models. To generate a model for studying the contribution of CCN2 expression in FoxD1-progenitor derived cells to development of fibrotic tissue remodeling, we set out to establish a FoxD1Cre - CCN2flox/flox mouse colony. However, all double-transgenic mice died soon after birth due to asphyxia. Histopathological examination revealed a reduction in alveolar space and lung weight, and subtle axial (thoracic and cervical) skeletal deformities. Together with the previously reported association of a FoxD1 containing locus with human adolescent idiopathic scoliosis, our data suggest that the development of fatal pulmonary hypoplasia caused by selective deletion of CCN2 from FoxD1-progenitor derived mesenchymal cells was secondary to aberrant axial skeletogenesis.



Similar content being viewed by others
Change history
14 April 2020
Pulmonary fibrosis is a severely disabling disease often leading to death. CCN2 (Cellular Communication Network factor 2, also known as CTGF) is a known mediator of fibrosis and clinical trials studying anti-CCN2 efficacy in pulmonary fibrosis are currently underway. Fork head box D1 (FoxD1) transcription factor is transiently expressed in several mesenchymal cell types, including those of fetal lungs. Differentiation of FoxD1-progenitor derived pericytes into myofibroblasts involves CCN2 expression and contributes importantly to maladaptive tissue remodeling in for example kidney and lung fibrosis models. To generate a model for studying the contribution of CCN2 expression in FoxD1-progenitor derived cells to development of fibrotic tissue remodeling, we set out to establish a FoxD1Cre - CCN2<Superscript>flox/flox</Superscript> mouse colony. However, all double-transgenic mice died soon after birth due to asphyxia. Histopathological examination revealed a reduction in alveolar space and lung weight, and subtle axial (thoracic and cervical) skeletal deformities. Together with the previously reported association of a FoxD1 containing locus with human adolescent idiopathic scoliosis, our data suggest that the fatal pulmonary hypoplasia resulting from selective deletion of CCN2 from FoxD1-progenitor derived mesenchymal cells developed secondary to impaired breathing movements due to aberrant axial skeletogenesis.
References
Baguma-Nibasheka M, Kablar B (2008) Pulmonary hypoplasia in the connective tissue growth factor (Ctgf) null mouse. Dev Dyn 237:485–493. https://doi.org/10.1002/dvdy.21433
Burgos CM, Nord M, Roos A, Didon L, Eklöf AC, Frenckner B (2010) Connective tissue growth factor expression pattern in lung development. Exp Lung Res 36:441–450. https://doi.org/10.3109/01902141003714056
Cameron TL, Belluoccio D, Farlie PG et al (2009) Global comparative transcriptome analysis of cartilage formation in vivo. BMC Dev Biol 9:20–17. https://doi.org/10.1186/1471-213X-9-20
Edery P, Margaritte-Jeannin P, Biot B, Labalme A, Bernard JC, Chastang J, Kassai B, Plais MH, Moldovan F, Clerget-Darpoux F (2011) New disease gene location and high genetic heterogeneity in idiopathic scoliosis. Eur J Hum Genet 19:865–869. https://doi.org/10.1038/ejhg.2011.31
Hall-Glenn F, De Young RA, Huang B-L et al (2012) CCN2/connective tissue growth factor is essential for pericyte adhesion and endothelial basement membrane formation during angiogenesis. PLoS One 7:e30562. https://doi.org/10.1371/journal.pone.0030562
Hung C, Linn G, Chow Y-H, Kobayashi A, Mittelsteadt K, Altemeier WA, Gharib SA, Schnapp LM, Duffield JS (2013) Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med 188:820–830. https://doi.org/10.1164/rccm.201212-2297OC
Inanlou MR, Baguma-Nibasheka M, Kablar B (2005) The role of fetal breathing-like movements in lung organogenesis. Histol Histopathol 20:1261–1266. https://doi.org/10.14670/HH-20.1261
Ivkovic S, Yoon BS, Popoff SN, et al (2003) Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development 130:2779–2791.
Kobayashi A, Mugford JW, Krautzberger AM, Naiman N, Liao J, McMahon A (2014) Identification of a multipotent self-renewing stromal progenitor population during mammalian kidney organogenesis. Stem Cell Rep 3:650–662. https://doi.org/10.1016/j.stemcr.2014.08.008
Kubota S, Takigawa M (2007) Role of CCN2/CTGF/Hcs24 in bone growth. Int Rev Cytol 257:1–41. https://doi.org/10.1016/S0074-7696(07)57001-4
Liu S, Shi-wen X, Abraham DJ, Leask A (2011) CCN2 is required for bleomycin-induced skin fibrosis in mice. Arthritis Rheum 63:239–246. https://doi.org/10.1002/art.30074
Pan LH, Yamauchi K, Uzuki M, Nakanishi T, Takigawa M, Inoue H, Sawai T (2001) Type II alveolar epithelial cells and interstitial fibroblasts express connective tissue growth factor in IPF. Eur Respir J 17:1220–1227. https://doi.org/10.1183/09031936.01.00074101
Rigueur D, Lyons KM (2014) Whole-mount skeletal staining. Methods Mol Biol 1130:113–121. https://doi.org/10.1007/978-1-62703-989-5_9
Shiwen X, Rajkumar V, Denton CP, Leask A, Abraham DJ (2009) Pericytes display increased CCN2 expression upon culturing. J Cell Commun Signal 3:61–64. https://doi.org/10.1007/s12079-009-0053-7
Wang X, Cui H, Wu S (2019) CTGF: A potential therapeutic target for Bronchopulmonary dysplasia. Eur J Pharmacol 860:172588. https://doi.org/10.1016/j.ejphar.2019.172588
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
(MP4 14.8 MB)
Rights and permissions
About this article
Cite this article
Falke, L.L., He, N., Chuva de Sousa Lopes, S.M. et al. FoxD1-driven CCN2 deletion causes axial skeletal deformities, pulmonary hypoplasia, and neonatal asphyctic death. J. Cell Commun. Signal. 13, 573–577 (2019). https://doi.org/10.1007/s12079-020-00549-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12079-020-00549-4