Abstract
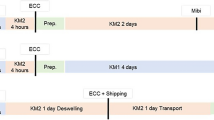
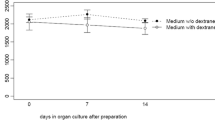
To determine the safety and graft quality of eye bank precut and preloaded grafts for Descemet membrane endothelial keratoplasty (DMEK) after storage and shipping in a novel preloaded transport cartridge compared to precut grafts in a conventional viewing chamber. In this laboratory proof-of-concept study, 29 human donor corneas that were unsuitable for transplantation with a mean endothelial cell density of 1948 ± 260 cells/mm2 were prepared using liquid bubble technique for producing precut lamellar grafts. The grafts were either preloaded into novel transport cartridge (n = 16) or transferred into conventional Krolman viewing chamber (control, n = 13). Grafts were stored for 24 or 48 h in dextran-containing medium at room temperature and subjected to a shipping simulation. Endothelial cell loss (ECL) and morphology were determined at different steps. Endothelial cell viability staining was performed with calcein dye. Mean ECL in the preloaded transport cartridge was 0.7% ± 1.2% after 24 h and 3.4% ± 1.2% (p = 0.006) after 48 h storage and injection. In the control group the ECL was mean 1.6% ± 2.7% after 24 h compared to 3.7% ± 0.9% (p = 0.042) after 48 h. The slightly higher endothelial cell loss in the viewing chamber group after 48 h was not statistically significant compared to the preloaded transport cartridge (p = 0.8). Calcein staining was comparably low in all groups and correlated with the low ECL in both groups. DMEK grafts can be preloaded into a novel transport cartridge using a "no touch" technique, stored and shipped for up to 2 days in dextran-containing medium without significant ECL.





Similar content being viewed by others
References
Abdin A, Daas L, Pattmöller M et al (2018) Negative impact of dextran in organ culture media for pre-stripped tissue preservation on DMEK (Descemet membrane endothelial keratoplasty) outcome. Graefes Arch Clin Exp Ophthalmol 256:2135–2142
Deng SX, Sanchez PJ, Chen L (2015) Clinical outcomes of Descemet membrane endothelial keratoplasty using eye bank-prepared tissues. Am J Ophthalmol 159:590–596
Feng MT, Burkhart ZN, Price FW et al (2013) Effect of donor preparation-to-use times on Descemet membrane endothelial keratoplasty outcomes. Cornea 32:1080–1082
Heinzelmann S, Böhringer D, Eberwein P et al (2017) Graft dislocation and graft failure following Descemet membrane endothelial keratoplasty (DMEK) using precut tissue: a retrospective cohort study. Graefes Arch Clin Exp Ophthalmol 255:127–133
Kim EC, Bonfadini G, Todd L et al (2014) Simple, inexpensive, and effective injector for Descemet membrane endothelial keratoplasty. Cornea 33:649–652
Kruse FE, Laaser K, Cursiefen C et al (2011) A stepwise approach to donor preparation and insertion increases safety and outcome of Descemet membrane endothelial keratoplasty. Cornea 30:580–587
Majmadur PA, Johnson L (2017) Enhancing DMEK success by identifying optimal levels of trypan blue dye application to donor corneal tissue. Cornea 36:217–221
Martin AI, Devasahayam R, Hodge C et al (2017) Analysis of the learning curve for pre-cut corneal specimens in preparation for lamellar transplantation: a prospective, single-centre, consecutive case series prepared at the Lions New South Wales Eye Bank. Clin Exp Ophthalmol 45:689–694
Marty AS, Burillon C, Desanlis A et al (2016) Validation of an endothelial roll preparation for Descemet membrane endothelial keratoplasty by a cornea bank using "no touch" dissection technique. Cell Tissue Bank 17:225–232
Newman LR, DeMill DL, Zeidenweber DA et al (2018) Preloaded descemet membrane endothelial keratoplasty donor tissue: surgical technique and early clinical results. Cornea 37:981–986
Parekh M, Ruzza A, Ferrari S et al (2016a) Preloaded tissues for Descemet membrane endothelial keratoplasty. Am J Ophthalmol 166:120–125
Parekh M, Ruzza A, Ferrari S et al (2016b) Preservation of preloaded DMEK lenticules in dextran and non-dextran-based organ culture medium. J Ophthalmol 2016:5830835
Price MO, Giebel AW, Fairchild KM et al (2009) Descemet’s membrane endothelial keratoplasty: prospective multicenter study of visual and refractive outcomes and endothelial survival. Ophthalmology 116:2361–2368
Regnier M, Auxenfans C, Maucort-Boulch D et al (2017) Eye bank prepared versus surgeon cut endothelial graft tissue for Descemet membrane endothelial keratoplasty: an observational study. Medicine (Baltimore) 96:e6885
Rickmann A, Wahl S, Katsen-Globa et al (2019) Safety analysis and results of a borosilicate glass cartridge for no-touch graft loading and injection in Descemet membrane endothelial keratoplasty. Int Ophthalmol 39:2295–2301
Romano V, Parekh M, Ruzza A et al (2018) Comparison of preservation and transportation protocols for preloaded Descemet membrane endothelial keratoplasty. Br J Ophthalmol 102:549–555
Szurman P, Januschowski K, Rickmann A et al (2016) Novel liquid bubble dissection technique for DMEK lenticule preparation. Graefes Arch Clin Exp Ophthalmol 254:1819–1823
Tenkman LR, Price FW, Price MO (2014) Descemet membrane endothelial keratoplasty donor preparation: navigating challenges and improving efficiency. Cornea 33:319–325
Terry MA, Straiko MD, Veldman PB et al (2015) Standardized DMEK technique: reducing complications using prestripped tissue, novel glass injector, and sulfur hexafluoride (SF6) gas. Cornea 34:845–852
Tran KD, Dye PK, Odell K et al (2017) Evaluation and quality assessment of prestripped, preloaded Descemet membrane endothelial keratoplasty grafts. Cornea 36:484–490
Wolle MA, DeMill DL, Johnson L et al (2017) Quantitative analysis of endothelial cell loss in preloaded descemet membrane endothelial keratoplasty grafts. Cornea 36:1295–1301
Yoeruek E, Hofmann J, Bartz-Schmidt KU (2013) Comparison of swollen and dextran deswollen organ-cultured corneas for Descemet membrane dissection preparation: histological and ultrastructural findings. Invest Ophthalmol Vis Sci 54:8036–8040
Yoeruek E, Bartz-Schmidt KU, Hofmann J (2016) Impact of the radius of the injector system on the cell viability in Descemet membrane endothelial. Acta Ophthalmol 94:e1–e5
Zeidenweber DA, Tran KD, Sales CS et al (2017) Prestained and preloaded DMEK grafts: an evaluation of tissue quality and stain retention. Cornea 36:1402–1407
Funding
This study was not funded.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Prof. Peter Szurman has a patent for a Device for preparing and introducing a transplant or an implant into a living body, in particular for ophthalmological interventions: EP2533724 B1; WO2012065602 A3. Prof. Peter Szurman has a pendant patent for the transport catridge: EP 3 046 509 A1. All other authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rickmann, A., Wahl, S., Hofmann, N. et al. Comparison of preloaded grafts for Descemet membrane endothelial keratoplasty (DMEK) in a novel preloaded transport cartridge compared to conventional precut grafts. Cell Tissue Bank 21, 205–213 (2020). https://doi.org/10.1007/s10561-020-09814-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-020-09814-7