Abstract
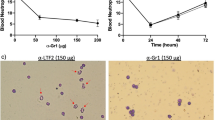
The chromoblastomycosis is a subcutaneous mycosis with a high morbidity rate, Fonsecaea pedrosoi being the largest etiologic agent of this mycosis, usually confined to the skin and subcutaneous tissues. Rarely people get the cure, because the therapies shown to be deficient and few studies report the host–parasite relationship. Dendritic cells (DCs) are specialized in presenting antigens to naïve T lymphocytes inducing primary immune responses. Therefore, we propose to study the migratory capacity of DCs after infection with conidia of F. pedrosoi. The phenotype of DCs was evaluated using cells obtained from footpad and lymph nodes of BALB/c mice after 12, 24 and 72 h of infection. After 24 and 72 h of infection, we found a significant decrease in DCs in footpad and a significant increase in the lymph nodes after 72 h. The expression of surface markers and co-stimulatory molecules were reduced in cells obtained from footpad. To better assess the migratory capacity of DCs migration from footpad, CFSE-stained conidia were injected subcutaneously. We found that after 12 and 72 h, CD11c+ cells were increased in regional lymph nodes, leading us to believe that DCs (CD11c+) were able to phagocytic conidia present in footpad and migrated to regional lymph nodes.






Similar content being viewed by others
References
Ameen M. Chromoblastomycosis: clinical presentation and management. Clin Exp Dermatol. 2009;34:849–54.
Krzysciak PM, Pindycka-Piaszczynska M, Piaszczynski M. Chromoblastomycosis. Postep Derm Alegol. 2014;5:310–21.
Queiroz-Telles F, Esterre P, Perez-Blanco M, Vitale RG, Salgado CG, Bonifaz A. Chromoblastomycosis: an overview of clinical manifestations, diagnosis and treatment. Med Mycol. 2009;49:3–15.
Ajello L. The gamut of human infections caused by dematiaceous fungi. Japanese J Med Mycol. 1981;22:1–5.
Castro LGM, de Andrade TS. Chromoblastomycosis: still a therapeutic challenge. Expert Rev Dermatol. 2010;5:433–43.
Schwartz R, Baran E. Chromoblastomycosis e-medicine from WebMD. 2010. Available: http://emedicine.medscape.com/article/1092695-overview.
D’Avila SCGP, Pagliari C, Duarte MIS. The cell-mediated immune reaction in the cutaneous lesion of chromoblastomycosis and their correlation with different clinical forms of the disease. Mycopathologia. 2002;156:51–60.
Gauthier GM. Dimorphism in fungal pathogens of mammals, plants, and insects. Plos Pathogen. 2015;11(2):e1004608.
Esterre P, Ravisse P, Plyrol S, et al. Immunopathologie de la lesion cutanee de chromomycose. J Mycol Med. 1991;1:201.
Sotto MN, De Brito T, Maria A, Silva G, Vidal M, Castro LGM. Antigen distribution and antigen-presenting cells in skin biopsies of human chromoblastomycosis. J Cutan Pathol. 2004;31:14–8.
Klechevsky E, Kato H, Sponaas A-M. Dendritic cells star in Vancouver. JEM. 2005;202:5–10.
Tassaneetrithep B, Burgess TH, Granelli-Piperno A, Trumpfheller C, Finke J, Sun W, et al. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J Exp Med J Exp. 2003;197:823–9.
Guermonprez P, Valladeau J, Zitvogel L, Théry C, Amigorena S. Antigen presentation and t cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–67.
Newman SL, Holly A. Candida albicans is phagocytosed, killed, and processed for antigen presentation by human dendritic cells. Infect Immun. 2001;69:6813–22.
Bozza S, Gaziano R, Spreca A, Bacci A, Montagnoli C, di Francesco, Romani L. Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus. J Immunol. 2002;168(3):1362–71.
Sousa MG, Eid E, Ghosn B, Nascimento RC, Bomfim GF, Noal V, et al. Monocyte-derived dendritic cells from patients with severe forms of chromoblastomycosis induce CD4 T cell activation in vitro. Clin Exp Immunol. 2009;156:117–25.
Gonzalez-Juarrero M, Orme IM. Characterization of murine lung dendritic cells infected with Mycobaterium tuberculosis. Infect Immunol. 2001;69:1127.
Li JLY, Ng GL. Peeking into the secret life of neutrophils. Immunol Res. 2012;53:168–81.
Rose S, Misharin A, Perlman H. A novel Ly6C/Ly6G-based strategy to analyze the mouse splenic myeloid compartment. Cytometry A. 2012;81(4):343–50.
Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic Dendrtic Cells. Ann Rev Immunol. 2003;21:685–711.
Jiang W, Swiggard WJ, Heufler C, Peng M, Mirza A, Steinman RM, et al. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375:151–5.
Vermaelen KY, Carro-Muino I, Lambrecht BN, Pauwels RA. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J Exp Med. 2001;193:51–60.
Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–52.
Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor. J Exp Med. 1994;179(4):1109–18.
Limongi CL, Alviano CS, de Souza W, Rozental S. Isolation and partial characterization of an adhesin from Fonsecaea pedrosoi. Med Myco. 2001;39:429–37.
Sousa MS, Reid DM, Schweighoffer E, Tybulewicz V, Ruland J, Langhorne J, Yamasaki S, Taylor FR, Almeida SR, Brown GD. Restoration of pattern recognition receptor coestimulation to treat chromoblastomycosis, a chronic fungal infection of the skin. Cell Host Microbe. 2011;9(5):436–43.
Cunha MM, Franzen AJ, Seabra SH, Herbst MH, Vugman NV, Borba LP, de Souza W, Rozental S. Melanin in Fonsecaea pedrosoi: a trap for oxidative radicals. BMC Microbiol. 2010;10:80.
Romero-Mastinez R, Wheeler M, Guerrero-Plata A, Rico G, Torres-Guerrero H. Biosynthesis and functions of melanin in Sporothrix schenckii. Infect Immun. 2000;68(6):3696–703.
Rosas AL, Nosanchuk JD, Gomez BL, Edens WA, Henson JM, Casadevall A. Isolation and serological analyses of fungal melanins. J Immunol Methods. 2000;244(1–2):69–80.
Gomez BL, Nosanchuk JD. Melanin and fungi. Curr Opin Infect Dis. 2003;16(2):91–6.
Nosanchuk JD, Casadevall A. Impact of melanin on microbial virulence and clinical resistance to antimicrobial components. Antimicrob Agents Chemother. 2006;50(11):3519–28.
Akoumianaki T, Kyrmizi I, Valsecchi I, et al. Aspergillus cell wall melanin blocks LC3-associated phagocytosis to promote pathogenicity. Cell Host Microb. 2016;19:79–90.
Rozental A, Alviano CS, Souza W. Fine structure and cytochemical study of the interaction between Fonsecaea pedrosoi and rat polymorphonuclear leukocyte. J Med Vet Mycol. 1996;34(5):323–30.
Nosanchuk JD, Rosas AL, Lee SC, Casadevall A. Melanisation of Cryptococcus neoformans in humam brains tisse. Lancet. 2000;355(9220):2049–50.
Bocca AL, Brito PP, Figueiredo F, Tosta CE. Inhibition of nitric oxide production by macrophages in chromoblastomycosis: a role for Fonsecaea pedrosoi melanin. Mycopathologia. 2006;161(4):195–203.
Acknowledgements
The present work was supported by FAPESP and Doctoral Fellowship from FAPESP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Handling Editor: Rosely Maria Zancope-Oliveira.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Telma Fátima Emidio Kimura and Lavínia Maria Dal’Mas Romera contributed equally to this work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
11046_2020_429_MOESM1_ESM.tif
F. pedrosoi conidia influences the number of Ly6G+ cells in footpad and lymph nodes. BALB/c mice were infected subcutaneously on the footpad with F. pedrosoi conidia. After 12, 24 and 72 h of infection, total cells were obtained from footpad and lymph nodes were collected +. As control, mice were infected with PBS. Data were analyzed by software FlowJo and gating strategy (FSc–SSc cell analysis and Ly6G+). All data are representative as mean of three independent experiments (*p < 0.01, SD). One-way ANOVA test and Tukey’s multiple comparison test. (A and B) Analysis of Ly6G+ in footpad. (C and D) Analysis of Ly6G+ in lymph nodes (TIFF 513 kb)
Rights and permissions
About this article
Cite this article
Kimura, T.F.E., Romera, L.M.D. & de Almeida, S.R. Fonsecaea pedrosoi Conidia Induces Activation of Dendritic Cells and Increases CD11c+ Cells in Regional Lymph Nodes During Experimental Chromoblastomycosis. Mycopathologia 185, 245–256 (2020). https://doi.org/10.1007/s11046-020-00429-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-020-00429-w