Abstract
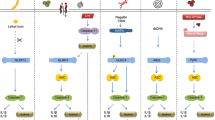
Pyroptosis is a programmed and inflammatory cell death initiated by inflammasome. During pyroptosis, cytosolic pattern recognition receptors, apoptosis-associated speck-like protein and pro-Caspase-1 form activated inflammasome together. Caspase-1 activated by inflammasome results in generating an N-terminal cleavage product of gasdermin D (GSDMD), which is a major executor of pyroptosis. As a consequence of pyroptosis, a large number of pro-inflammatory cytokines are released including IL-1β and IL-18. Nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) and absent in melanoma 2 (AIM2)-like receptors (ALRs) belong to cytosolic pattern recognition receptors and assemble inflammasomes by detecting host cell damage signals. Pyroptosis pathways are divided into canonical and non-canonical pathways according to the identification of damage signals by cytoplasmic protein sensors. Pyroptosis not only plays an important role in infection, but also plays a vital role in inflammatory immune diseases. This article reviews the advances research of pyroptosis initiated by inflammasome in inflammatory and immune diseases.


Similar content being viewed by others
References
Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8(11):1812–25. https://doi.org/10.1111/j.1462-5822.2006.00751.x.
McKenzie BA, Mamik MK, Saito LB, Boghozian R, Monaco MC, Major EO, et al. Caspase-1 inhibition prevents glial inflammasome activation and pyroptosis in models of multiple sclerosis. Proc Natl Acad Sci USA. 2018;115(26):E6065–E60746074. https://doi.org/10.1073/pnas.1722041115.
Ge X, Li W, Huang S, Yin Z, Xu X, Chen F, et al. The pathological role of NLRs and AIM2 inflammasome-mediated pyroptosis in damaged blood-brain barrier after traumatic brain injury. Brain Res. 2018;1697:10–20. https://doi.org/10.1016/j.brainres.2018.06.008.
Lee S, Hirohama M, Noguchi M, Nagata K, Kawaguchi A. Influenza A virus infection triggers pyroptosis and apoptosis of respiratory epithelial cells through the type I interferon signaling pathway in a mutually exclusive manner. J Virol. 2018. https://doi.org/10.1128/JVI.00396-18.
Guo Q, Wu Y, Hou Y, Liu Y, Liu T, Zhang H, et al. Cytokine secretion and pyroptosis of thyroid follicular cells mediated by enhanced NLRP3, NLRP1, NLRC4, and AIM2 inflammasomes are associated with autoimmune thyroiditis. Front Immunol. 2018;9:1197. https://doi.org/10.3389/fimmu.2018.01197.
Wang Y, Jia L, Shen J, Wang Y, Fu Z, Su S-A, et al. Cathepsin B aggravates coxsackievirus B3-induced myocarditis through activating the inflammasome and promoting pyroptosis. PLoS Pathog. 2018;14(1):e1006872. https://doi.org/10.1371/journal.ppat.1006872.
Zhang L, Huang Z, Xing R, Li X, Yin S, Mao J, et al. Increased HIF-1α in knee osteoarthritis aggravate synovial fibrosis via fibroblast-like synoviocyte pyroptosis. Oxid Med Cell Longev. 2019;2019:6326517. https://doi.org/10.1155/2019/6326517.
Schmid-Burgk JL, Chauhan D, Schmidt T, Ebert TS, Reinhardt J, Endl E, Hornung V. A genome-wide CRISPR (clustered regularly interspaced short palindromic repeats) screen identifies NEK7 as an essential component of NLRP3 inflammasome activation. J Biol Chem. 2016;291(1):103–9. https://doi.org/10.1074/jbc.C115.700492.
Pan J, Han L, Guo J, Wang X, Liu D, Tian J, et al. AIM2 accelerates the atherosclerotic plaque progressions in ApoE-/- mice. Biochem Biophys Res Commun. 2018;498(3):487–94. https://doi.org/10.1016/j.bbrc.2018.03.005.
Tan M-S, Tan L, Jiang T, Zhu X-C, Wang H-F, Jia C-D, Yu J-T. Amyloid-β induces NLRP1-dependent neuronal pyroptosis in models of Alzheimer’s disease. Cell Death Dis. 2014;5:e1382. https://doi.org/10.1038/cddis.2014.348.
Kovarova M, Hesker PR, Jania L, Nguyen M, Snouwaert JN, Xiang Z, et al. NLRP1-dependent pyroptosis leads to acute lung injury and morbidity in mice. J Immunol. 2012;189(4):2006–16. https://doi.org/10.4049/jimmunol.1201065.
Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38(2):240–4. https://doi.org/10.1038/ng1724.
Masters SL, Gerlic M, Metcalf D, Preston S, Pellegrini M, O’Donnell JA, et al. NLRP1 inflammasome activation induces pyroptosis of hematopoietic progenitor cells. Immunity. 2012;37(6):1009–233. https://doi.org/10.1016/j.immuni.2012.08.027.
Pétrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14(9):1583–9. https://doi.org/10.1038/sj.cdd.4402195.
Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278(11):8869–72. https://doi.org/10.1074/jbc.C200651200.
Faustin B, Lartigue L, Bruey J-M, Luciano F, Sergienko E, Bailly-Maitre B, et al. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell. 2007;25(5):713–24. https://doi.org/10.1016/j.molcel.2007.01.032.
Zhao D, Wu Y, Zhuang J, Xu C, Zhang F. Activation of NLRP1 and NLRP3 inflammasomes contributed to cyclic stretch-induced pyroptosis and release of IL-1β in human periodontal ligament cells. Oncotarget. 2016;7(42):68292–302. https://doi.org/10.18632/oncotarget.11944.
Liao H, Wang H, Rong X, Li E, Xu R-H, Peng Y. Mesenchymal stem cells attenuate radiation-induced brain injury by inhibiting microglia pyroptosis. Biomed Res Int. 2017;2017:1948985. https://doi.org/10.1155/2017/1948985.
Zhang B, Wei W, Qiu J. ALK is required for NLRP3 inflammasome activation in macrophages. Biochem Biophys Res Commun. 2018;501(1):246–52. https://doi.org/10.1016/j.bbrc.2018.04.226.
Luo B, Huang F, Liu Y, Liang Y, Wei Z, Ke H, et al. NLRP3 inflammasome as a molecular marker in diabetic cardiomyopathy. Front Physiol. 2017;8:519. https://doi.org/10.3389/fphys.2017.00519.
He Y, Zeng MY, Yang D, Motro B, Núñez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016;530(7590):354–7. https://doi.org/10.1038/nature16959.
Zhang Y, Lv X, Hu Z, Ye X, Zheng X, Ding Y, et al. Protection of Mcc950 against high-glucose-induced human retinal endothelial cell dysfunction. Cell Death Dis. 2017;8(7):e2941. https://doi.org/10.1038/cddis.2017.308.
Tang T, Lang X, Xu C, Wang X, Gong T, Yang Y, et al. CLICs-dependent chloride efflux is an essential and proximal upstream event for NLRP3 inflammasome activation. Nat Commun. 2017;8(1):202. https://doi.org/10.1038/s41467-017-00227-x.
Wu X, Ren G, Zhou R, Ge J, Chen F-H. The role of Ca2+ in acid-sensing ion channel 1a-mediated chondrocyte pyroptosis in rat adjuvant arthritis. Lab Invest. 2019;99(4):499–513. https://doi.org/10.1038/s41374-018-0135-3.
Zhang X, Luan J, Chen W, Fan J, Nan Y, Wang Y, et al. Mesoporous silica nanoparticles induced hepatotoxicity via NLRP3 inflammasome activation and caspase-1-dependent pyroptosis. Nanoscale. 2018;10(19):9141–52. https://doi.org/10.1039/c8nr00554k.
Wu X, Zhang H, Qi W, Zhang Y, Li J, Li Z, et al. Nicotine promotes atherosclerosis via ROS-NLRP3-mediated endothelial cell pyroptosis. Cell Death Dis. 2018;9(2):171. https://doi.org/10.1038/s41419-017-0257-3.
Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10(3):210–5. https://doi.org/10.1038/nri2725.
Tan C-C, Zhang J-G, Tan M-S, Chen H, Meng D-W, Jiang T, et al. NLRP1 inflammasome is activated in patients with medial temporal lobe epilepsy and contributes to neuronal pyroptosis in amygdala kindling-induced rat model. J Neuroinflammation. 2015;12:18. https://doi.org/10.1186/s12974-014-0233-0.
Ceballos-Olvera I, Sahoo M, Miller MA, Del Barrio L, Re F. Inflammasome-dependent pyroptosis and IL-18 protect against Burkholderia pseudomallei lung infection while IL-1β is deleterious. PLoS Pathog. 2011;7(12):e1002452. https://doi.org/10.1371/journal.ppat.1002452.
Byrne BG, Dubuisson J-F, Joshi AD, Persson JJ, Swanson MS. Inflammasome components coordinate autophagy and pyroptosis as macrophage responses to infection. MBio. 2013;4(1):e00620–e712. https://doi.org/10.1128/mBio.00620-12.
Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11(12):1136–42. https://doi.org/10.1038/ni.1960.
Maltez VI, Tubbs AL, Cook KD, Aachoui Y, Falcone EL, Holland SM, et al. Inflammasomes coordinate pyroptosis and natural killer cell cytotoxicity to clear infection by a ubiquitous environmental bacterium. Immunity. 2015;43(5):987–97. https://doi.org/10.1016/j.immuni.2015.10.010.
Zhao Y, Shao F. The NAIP-NLRC4 inflammasome in innate immune detection of bacterial flagellin and type III secretion apparatus. Immunol Rev. 2015;265(1):85–102. https://doi.org/10.1111/imr.12293.
Zhang L, Chen S, Ruan J, Wu J, Tong AB, Yin Q, et al. Cryo-EM structure of the activated NAIP2-NLRC4 inflammasome reveals nucleated polymerization. Science. 2015;350(6259):404–9. https://doi.org/10.1126/science.aac5789.
Hu Z, Zhou Q, Zhang C, Fan S, Cheng W, Zhao Y, et al. Structural and biochemical basis for induced self-propagation of NLRC4. Science. 2015;350(6259):399–404. https://doi.org/10.1126/science.aac5489.
Ryu J-C, Kim M-J, Kwon Y, Oh J-H, Yoon SS, Shin SJ, et al. Neutrophil pyroptosis mediates pathology of P. aeruginosa lung infection in the absence of the NADPH oxidase NOX2. Mucosal Immunol. 2017;10(3):757–74. https://doi.org/10.1038/mi.2016.73.
Choubey D, Panchanathan R. Absent in melanoma 2 proteins in SLE. Clin Immunol. 2017;176:42–8. https://doi.org/10.1016/j.clim.2016.12.011.
Yogarajah T, Ong KC, Perera D, Wong KT. AIM2 inflammasome-mediated pyroptosis in enterovirus A71-infected neuronal cells restricts viral replication. Sci Rep. 2017;7(1):5845. https://doi.org/10.1038/s41598-017-05589-2.
Costa Franco MMS, Marim FM, Alves-Silva J, Cerqueira D, Rungue M, Tavares IP, Oliveira SC. AIM2 senses Brucella abortus DNA in dendritic cells to induce IL-1β secretion, pyroptosis and resistance to bacterial infection in mice. Microbes Infect. 2019;21(2):85–93. https://doi.org/10.1016/j.micinf.2018.09.001.
Sagulenko V, Vitak N, Vajjhala PR, Vince JE, Stacey KJ. Caspase-1 is an apical caspase leading to caspase-3 cleavage in the AIM2 inflammasome response, independent of caspase-8. J Mol Biol. 2018;430(2):238–47. https://doi.org/10.1016/j.jmb.2017.10.028.
Eichholz K, Bru T, Tran TTP, Fernandes P, Welles H, Mennechet FJD, et al. Immune-complexed adenovirus induce AIM2-mediated pyroptosis in human dendritic cells. PLoS Pathog. 2016;12(9):e1005871. https://doi.org/10.1371/journal.ppat.1005871.
Meunier E, Wallet P, Dreier RF, Costanzo S, Anton L, Rühl S, et al. Guanylate-binding proteins promote activation of the AIM2 inflammasome during infection with Francisella novicida. Nat Immunol. 2015;16(5):476–84. https://doi.org/10.1038/ni.3119.
Woo S-R, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MYK, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41(5):830–42. https://doi.org/10.1016/j.immuni.2014.10.017.
Zhu Q, Zheng M, Balakrishnan A, Karki R, Kanneganti T-D. Gasdermin D promotes AIM2 inflammasome activation and is required for host protection against Francisella novicida. J Immunol. 2018;201(12):3662–8. https://doi.org/10.4049/jimmunol.1800788.
Man SM, Karki R, Sasai M, Place DE, Kesavardhana S, Temirov J, et al. IRGB10 liberates bacterial ligands for sensing by the AIM2 and caspase-11-NLRP3 inflammasomes. Cell. 2016;167(2):382–396.e17. https://doi.org/10.1016/j.cell.2016.09.012.
Corrales L, Woo S-R, Williams JB, McWhirter SM, Dubensky TW, Gajewski TF. Antagonism of the STING pathway via activation of the AIM2 inflammasome by intracellular DNA. J Immunol. 2016;196(7):3191–8. https://doi.org/10.4049/jimmunol.1502538.
Liu BC, Sarhan J, Panda A, Muendlein HI, Ilyukha V, Coers J, et al. Constitutive interferon maintains GBP expression required for release of bacterial components upstream of pyroptosis and anti-DNA responses. Cell Rep. 2018;24(1):155–168.e5. https://doi.org/10.1016/j.celrep.2018.06.012.
Yuan J, Zhu M, Deng S, Fan S, Xu H, Liao J, et al. Classical swine fever virus induces pyroptosis in the peripheral lymphoid organs of infected pigs. Virus Res. 2018;250:37–42. https://doi.org/10.1016/j.virusres.2018.04.004.
Mandal P, Feng Y, Lyons JD, Berger SB, Otani S, DeLaney A, et al. Caspase-8 collaborates with caspase-11 to drive tissue damage and execution of endotoxic shock. Immunity. 2018;49(1):42–55.e6. https://doi.org/10.1016/j.immuni.2018.06.011.
Lacey CA, Mitchell WJ, Dadelahi AS, Skyberg JA. Caspase-1 and caspase-11 mediate pyroptosis, inflammation, and control of brucella joint infection. Infect Immun. 2018. https://doi.org/10.1128/IAI.00361-18.
Ritter JL, Genco CA. Neisseria gonorrhoeae-induced inflammatory pyroptosis in human macrophages is dependent on intracellular gonococci and lipooligosaccharide. J Cell Death. 2018;11:1179066017750902. https://doi.org/10.1177/1179066017750902.
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–5. https://doi.org/10.1038/nature15514.
Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526(7575):666–71. https://doi.org/10.1038/nature15541.
Ran S, Chu M, Gu S, Wang J, Liang J. Enterococcus faecalis induces apoptosis and pyroptosis of human osteoblastic MG63 cells via the NLRP3 inflammasome. Int Endod J. 2019;52(1):44–53. https://doi.org/10.1111/iej.12965.
Nascimento DO, Vieira-de-Abreu A, Arcanjo AF, Bozza PT, Zimmerman GA, Castro-Faria-Neto HC. Integrin αDβ2 (CD11d/CD18) modulates leukocyte accumulation, pathogen clearance, and pyroptosis in experimental salmonella typhimurium infection. Front Immunol. 2018;9:1128. https://doi.org/10.3389/fimmu.2018.01128.
Rashad S, Niizuma K, Sato-Maeda M, Fujimura M, Mansour A, Endo H, et al. Early BBB breakdown and subacute inflammasome activation and pyroptosis as a result of cerebral venous thrombosis. Brain Res. 2018;1699:54–68. https://doi.org/10.1016/j.brainres.2018.06.029.
Lin X, Ye H, Siaw-Debrah F, Pan S, He Z, Ni H, et al. AC-YVAD-CMK inhibits pyroptosis and improves functional outcome after intracerebral hemorrhage. Biomed Res Int. 2018;2018:3706047. https://doi.org/10.1155/2018/3706047.
Wang X, Pan J, Liu H, Zhang M, Liu D, Lu L, et al. AIM2 gene silencing attenuates diabetic cardiomyopathy in type 2 diabetic rat model. Life Sci. 2019;221:249–58. https://doi.org/10.1016/j.lfs.2019.02.035.
Yang F, Qin Y, Lv J, Wang Y, Che H, Chen X, et al. Silencing long non-coding RNA Kcnq1ot1 alleviates pyroptosis and fibrosis in diabetic cardiomyopathy. Cell Death Dis. 2018;9(10):1000. https://doi.org/10.1038/s41419-018-1029-4.
Mathews RJ, Robinson JI, Battellino M, Wong C, Taylor JC, Eyre S, et al. Evidence of NLRP3-inflammasome activation in rheumatoid arthritis (RA); genetic variants within the NLRP3-inflammasome complex in relation to susceptibility to RA and response to anti-TNF treatment. Ann Rheum Dis. 2014;73(6):1202–10. https://doi.org/10.1136/annrheumdis-2013-203276.
Zhao J, Wang H, Dai C, Wang H, Zhang H, Huang Y, et al. P2X7 blockade attenuates murine lupus nephritis by inhibiting activation of the NLRP3/ASC/caspase 1 pathway. Arthritis Rheum. 2013;65(12):3176–85. https://doi.org/10.1002/art.38174.
Ma Z-Z, Sun H-S, Lv J-C, Guo L, Yang Q-R. Expression and clinical significance of the NEK7-NLRP3 inflammasome signaling pathway in patients with systemic lupus erythematosus. J Inflamm (Lond). 2018;15:16. https://doi.org/10.1186/s12950-018-0192-9.
Vakrakou AG, Boiu S, Ziakas PD, Xingi E, Boleti H, Manoussakis MN. Systemic activation of NLRP3 inflammasome in patients with severe primary Sjögren’s syndrome fueled by inflammagenic DNA accumulations. J Autoimmun. 2018;91:23–33. https://doi.org/10.1016/j.jaut.2018.02.010.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (No. U1803129, 81803538 and 81673444).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Dr. John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liang, F., Zhang, F., Zhang, L. et al. The advances in pyroptosis initiated by inflammasome in inflammatory and immune diseases. Inflamm. Res. 69, 159–166 (2020). https://doi.org/10.1007/s00011-020-01315-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-020-01315-3