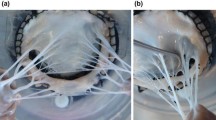
Abstract
Proper blood flow through the atrioventricular heart valves (AHVs) relies on the holistic function of the valve and subvalvular structures, and a failure of any component can lead to life-threatening heart disease. A comprehension of the mechanical characteristics of healthy valvular components is necessary for the refinement of heart valve computational models. In previous studies, the chordae tendineae have been mechanically characterized as individual structures, usually in a clamping-based approach, which may not accurately reflect the in vivo chordal interactions with the leaflet insertion and papillary muscles. In this study, we performed uniaxial mechanical testing of strut chordae tendineae of the AHVs under a unique tine-based leaflet–chordae–papillary muscle testing to observe the chordae mechanics while preserving the subvalvular component interactions. Results of this study provided insight to the disparity of chordae tissue stress-stretch responses between the mitral valve (MV) and the tricuspid valve (TV) under their respective emulated physiological loading. Specifically, strut chordae tendineae of the MV anterior leaflet had peak stretches of 1.09–1.16, while peak stretches of 1.08–1.11 were found for the TV anterior leaflet strut chordae. Constitutive parameters were also derived for the chordae tissue specimens using an Ogden model, which is useful for AHV computational model refinement. Results of this study are beneficial to the eventual improvement of treatment methods for valvular disease.







Similar content being viewed by others
References
Billiar, K. L., and M. S. Sacks. A method to quantify the fiber kinematics of planar tissues under biaxial stretch. J. Biomech. 30:753–756, 1997.
Chuong, C.-J., and Y.-C. Fung. Residual stress in arteries. In: Frontiers in Biomechanics, edited by G. W. Schmid-Schönbein, S. L.-Y. Woo, and B. W. Zweifach. New York: Springer, 1986, pp. 117–129.
Cochran, R. P., and K. S. Kuzelman. Comparison of viscoelastic properties of suture versus porcine mitral valve chordae tendineae. J. Card. Surg. 6:508–513, 1991.
Colli, A., E. Manzan, K. Rucinskas, V. Janusauskas, F. Zucchetta, D. Zakarkaitė, A. Aidietis, and G. Gerosa. Acute safety and efficacy of the NeoChord procedure. Interact. Cardiovasc. Thorac. Surg. 20:575–581, 2015.
Eckert, C. E., B. Zubiate, M. Vergnat, J. H. Gorman, R. C. Gorman, and M. S. Sacks. In vivo dynamic deformation of the mitral valve annulus. Ann. Biomed. Eng. 37:1757–1771, 2009.
Foutz, T. L., E. A. Stone, and C. F. Abrams, III. Effects of freezing on mechanical properties of rat skin. Am. J. Vet. Res. 53:788–792, 1992.
Goth, W., S. Potter, A. C. B. Allen, J. Zoldan, M. S. Sacks, and J. W. Tunnell. Non-destructive reflectance mapping of collagen fiber alignment in heart valve leaflets. Ann. Biomed. Eng. 47:1250–1264, 2019.
Gunning, G. M., and B. P. Murphy. Characterisation of the fatigue life, dynamic creep and modes of damage accumulation within mitral valve chordae tendineae. Acta Biomater. 24:193–200, 2015.
Hosseini, S. M., W. Wilson, K. Ito, and C. C. van Donkelaar. How preconditioning affects the measurement of poro-viscoelastic mechanical properties in biological tissues. Biomech. Model. Mechanobiol. 13:503–513, 2014.
Humphrey, J. D., D. L. Vawter, and R. P. Vito. Quantification of strains in biaxially tested soft tissues. J. Biomech. 20:59–65, 1987.
Jett, S. V., L. T. Hudson, R. Baumwart, B. N. Bohnstedt, A. Mir, H. M. Burkhart, G. A. Holzapfel, Y. Wu, and C.-H. Lee. Integration of polarized spatial frequency domain imaging (pSFDI) with a biaxial mechanical testing system for quantification of load-dependent collagen architecture in soft collagenous tissues. Acta Biomater. 102:149–168, 2020.
Jimenez, J. H., D. D. Soerensen, Z. He, S. He, and A. P. Yoganathan. Effects of a saddle shaped annulus on mitral valve function and chordal force distribution: An in vitro study. Ann. Biomed. Eng. 31:1171–1181, 2003.
Khoiy, K. A., and R. Amini. On the biaxial mechanical response of porcine tricuspid valve leaflets. J. Biomech. Eng. 138:104504, 2016.
Klabunde, R. Cardiovascular Physiology Concepts. Philadelphia: Lippincott Williams & Wilkins, 2011.
Lam, J. H. C., N. Ranganathan, E. D. Wigle, and M. D. Silver. Morphology of the human mitral valve. Circulation 41:449–458, 1970.
Lee, C.-H., J.-P. Rabbah, A. P. Yoganathan, R. C. Gorman, J. H. Gorman, III, and M. S. Sacks. On the effects of leaflet microstructure and constitutive model on the closing behavior of the mitral valve. Biomech. Model. Mechanobiol. 14:1281–1302, 2015.
Liao, J., and I. Vesely. A structural basis for the size-related mechanical properties of mitral valve chordae tendineae. J. Biomech. 36:1125–1133, 2003.
Lomholt, M., S. L. Nielsen, S. Hansen, N. T. Andersen, and J. M. Hasenkam. Differential tension between secondary and primary mitral chordae in an acute in-vivo porcine model. J. Heart Valve Dis. 11:337–345, 2002.
Ogden, R. Elastic deformations of rubberlike solids. In: Mechanics of Solids, edited by H. G. Hopkins. Amsterdam: Elsevier, 1982, pp. 499–537.
Phillips, M. R., R. C. Daly, H. V. Schaff, J. A. Dearani, C. J. Mullany, and T. A. Orszulak. Repair of anterior leaflet mitral valve prolapse: Chordal replacement versus chordal shortening. Ann. Thorac. Surg. 69:25–29, 2000.
Pokutta-Paskaleva, A., F. Sulejmani, M. DelRocini, and W. Sun. Comparative mechanical, morphological, and microstructural characterization of porcine mitral and tricuspid leaflets and chordae tendineae. Acta Biomater. 85:241–252, 2019.
Prot, V., B. Skallerud, G. Sommer, and G. A. Holzapfel. On modelling and analysis of healthy and pathological human mitral valves: Two case studies. J. Mech. Behav. Biomed. Mater. 3:167–177, 2010.
Rabbah, J.-P., N. Saikrishnan, and A. P. Yoganathan. A novel left heart simulator for the multi-modality characterization of native mitral valve geometry and fluid mechanics. Ann. Biomed. Eng. 41:305–315, 2013.
Ritchie, J., J. Jimenez, Z. He, M. S. Sacks, and A. P. Yoganathan. The material properties of the native porcine mitral valve chordae tendineae: An in vitro investigation. J. Biomech. 39:1129–1135, 2006.
Sedransk, K. L., K. J. Grande-Allen, and I. Vesely. Failure mechanics of mitral valve chordae tendineae. J. Heart Valve Dis. 11:644–650, 2002.
Shah, P. M. Current concepts in mitral valve prolapse—Diagnosis and management. J. Cardiol. 56:125–133, 2010.
Silver, M. D., J. H. C. Lam, N. Ranganathan, and E. D. Wigle. Morphology of the human tricuspid valve. Circulation 43:333–348, 1971.
Smedira, N. G., R. Selman, D. M. Cosgrove, P. M. McCarthy, B. W. Lytle, P. C. Taylor, C. Apperson-Hansen, R. W. Stewart, and F. D. Loop. Repair of anterior leaflet prolapse: Chordal transfer is superior to chordal shortening. J. Thorac. Cardiovasc. Surg. 112:287–292, 1996.
Sousa, U. M., P. Grare, V. Jebara, J. F. Fuzelier, M. Portoghese, C. Acar, J. Relland, S. Mihaileanu, J. N. Fabiani, and A. Carpentier. Transposition of chordae in mitral valve repair. Mid-term results. Circulation 88:II35–II38, 1993.
Stemper, B. D., N. Yoganandan, M. R. Stineman, T. A. Gennarelli, J. L. Baisden, and F. A. Pintar. Mechanics of fresh, refrigerated, and frozen arterial tissue. J. Surg. Res. 139:236–242, 2007.
Storn, R., and K. Price. Differential evolution—A simple and efficient heuristic for global optimization over continuous spaces. J. Global Opt. 11:341–359, 1997.
Tabata, M., H. Kasegawa, T. Fukui, A. Shimizu, Y. Sato, and S. Takanashi. Long-term outcomes of artificial chordal replacement with tourniquet technique in mitral valve repair: A single-center experience of 700 cases. J. Thorac. Cardiovasc. Surg. 148:2033–2038, 2014.
Waller, B. F., J. Howard, and S. Fess. Pathology of tricuspid valve stenosis and pure tricuspid regurgitation—Part I. Clin. Cardiol. 18:97–102, 1995.
Zuo, K., T. Pham, K. Li, C. Martin, Z. He, and W. Sun. Characterization of biomechanical properties of aged human and ovine mitral valve chordae tendineae. J. Mech. Behav. Biomed. Mater. 62:607–618, 2016.
Acknowledgments
Supports from the American Heart Association Scientist Development Grant (SDG) Award (16SDG27760143) and the Presbyterian Health Foundation Team Science Grants (C5122401) are gratefully acknowledged. CHL was in part supported by the institutional start-up funds from the School of Aerospace and Mechanical Engineering (AME), the IBEST-OUHSC Funding for Interdisciplinary Research, and the research funding through the Faculty Investment Program from the Research Council at the University of Oklahoma (OU). CR and DL were supported by the Mentored Research Fellowship from the Office of Undergraduate Research and the Undergraduate Research Opportunities Program from the Honors College at OU.
Conflicts of interest
The authors of this paper have no financial or personal relationships with other people or organizations that could inappropriately influence (bias) our work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Peter E. McHugh oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix: Polarized Spatial Frequency Domain Imaging (pSFDI) for Quantification of the Collagen Fiber Architecture of the Chordae–Leaflet Insertion Region
Appendix: Polarized Spatial Frequency Domain Imaging (pSFDI) for Quantification of the Collagen Fiber Architecture of the Chordae–Leaflet Insertion Region
In this section, we briefly discuss about the imaging method used to obtain the collagen fiber orientations of the chordae-leaflet insertion region, as described in “Potential Advantages of the Proposed CT-Entity Testing Method” section. The polarized spatial frequency domain imaging (pSFDI) technique takes advantage of the polarized light-dependent birefringent properties of the collagen fibers. This birefringent property of the collagen fibers can be used in conjunction with a polarizer, a camera, and a light projector to quantify the collagen fiber orientations based on the intensity of reflected light from the fiber. The imaging is performed in the sequential order (see Figs. 1 and 2 of Jett et al.11):
- (i)
Projected incident light through a polarizer at an angle θpolarizer onto the tissue specimen
- (ii)
The incident light is reflected from the collagen fibers
- (iii)
The reflected light passes through a polarizer at the same θpolarizer as in step (i)
- (iv)
The light intensity at the specific θpolarizer is captured by a camera
This imaging procedure is conducted for θpolarizer ranging from 0° to 180° (at an increment of 5°) to capture the intensity of the collagen fibers. Owing to the birefringent response of the collagen fiber networks, the peak intensity of reflected light represents the quantified collagen fiber orientation angle θpolarizer. In addition, the captured light intensity, say Iout, can be described by the following three-term Fourier series:
where τsys represents the non-birefringent intensities, and a0, a2, and a4 are the three Fourier coefficients. Specifically, a0 describes the mean reflected light intensity from the sample, whereas a2 and a4 represent the optical anisotropies arising from the sample’s birefringence.
After obtaining the orientation angle of the collagen fibers, the degree of fiber alignment can be quantified through an optics-based metric – the degree of optical anisotropy (DOA):
The technology also allows for investigating the collagen fiber orientation and dispersion at varied depths of the tissue based on the spatial frequency domain imaging (SFDI) theory. However, the preliminary result presented in this study (Fig. 5c) only contains the tissues’ collagen fiber architecture through the full tissue depth. For more details about the SFDI theory, the pSFDI imaging procedures, and the corresponding data analyses, the reader is referred to Jett et al.11 and Goth et al.7
Rights and permissions
About this article
Cite this article
Ross, C.J., Laurence, D.W., Hsu, MC. et al. Mechanics of Porcine Heart Valves’ Strut Chordae Tendineae Investigated as a Leaflet–Chordae–Papillary Muscle Entity. Ann Biomed Eng 48, 1463–1474 (2020). https://doi.org/10.1007/s10439-020-02464-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-020-02464-6