Abstract
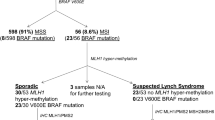
Routine diagnostics for colorectal cancer patients suspected of having Lynch-Syndrome (LS) currently uses Next-Generation-Sequencing (NGS) of targeted regions within the DNA mismatch repair (MMR) genes. This analysis can reliably detect nucleotide alterations and copy-number variations (CNVs); however, CNV-neutral rearrangements comprising gene inversions or large intronic insertions remain undetected because their breakpoints are usually not covered. As several founder mutations exist for LS, we established PCR-based screening methods for five known rearrangements in MLH1, MSH2, or PMS2, and investigated their prevalence in 98 German patients with suspicion of LS without a causative germline variant or CNV detectable in the four MMR genes. We found no recurrence of CNV-neutral structural rearrangements previously described: Neither for two inversions in MLH1 (exon 1 and exon 16–19) within 33 MLH1-deficient patients, nor for two inversions in MSH2 (exon 1–7 and exon 2–6) within 48 MSH2-deficient patients. The PMS2 insertion in intron 7 was detected in one of 17 PMS2-deficient patients. None of the four genomic inversions constitutes a founder event within the German population, but we advise to test the rare cases with unsolved PMS2-deficiency upon the known insertion. As a next diagnostic step, tumour tissue of the unsolved patients should be sequenced for somatic variants, and germline analysis of additional genes with an overlapping clinical phenotype should be considered. Alternatively, full-length cDNA analyses may detect concealed MMR-defects in cases with family history.

Similar content being viewed by others
Abbreviations
- CNV(s):
-
Copy-number variation(s)
- CRC:
-
Colorectal cancer
- LS:
-
Lynch-syndrome
- MLPA:
-
Multiplex ligation-dependent probe amplification
- MMR:
-
DNA mismatch repair
- NGS:
-
Next-generation-sequencing
- PCR:
-
Polymerase chain reaction
- SNV:
-
Single nucleotide variant
- SVA:
-
SINE–VNTR–Alu
- VUS:
-
Variant of uncertain significance
References
Lynch HT, de la Chapelle A (1999) Genetic susceptibility to non-polyposis colorectal cancer. J Med Genet 36(11):801–818
Deng G, Bell I, Crawley S, Gum J, Terdiman JP, Allen BA, Truta B, Sleisenger MH, Kim YS (2004) BRAF mutation is frequently present in sporadic colorectal cancer with methylated hMLH1, but not in hereditary nonpolyposis colorectal cancer. Clin Cancer Res 10:191–195
Pearlman R, Haraldsdottir S, de la Chapelle A, Jonasson JG, Liyanarachchi S, Frankel WL, Rafnar T, Stefansson K, Pritchard CC, Hampel H (2019) Clinical characteristics of patients with colorectal cancer with double somatic mismatch repair mutations compared with Lynch syndrome. J Med Genet 56(7):462–470. https://doi.org/10.1136/jmedgenet-2018-105698
Vargas-Parra GM, Gonzalez-Acosta M, Thompson BA, Gomez C, Fernandez A, Damaso E, Pons T, Morak M, Del Valle J, Iglesias S, Velasco A, Solanes A, Sanjuan X, Padilla N, de la Cruz X, Valencia A, Holinski-Feder E, Brunet J, Feliubadalo L, Lazaro C, Navarro M, Pineda M, Capella G (2017) Elucidating the molecular basis of MSH2-deficient tumors by combined germline and somatic analysis. Int J Cancer 141(7):1365–1380. https://doi.org/10.1002/ijc.30820
Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J, Fishel R, Lindor NM, Burgart LJ, Hamelin R, Hamilton SR, Hiatt RA, Jass J, Lindblom A, Lynch HT, Peltomaki P, Ramsey SD, Rodriguez-Bigas MA, Vasen HF, Hawk ET, Barrett JC, Freedman AN, Srivastava S (2004) Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 96(4):261–268
Vasen HF, Watson P, Mecklin JP, Lynch HT (1999) New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 116(6):1453–1456
Grabowski M, Mueller-Koch Y, Grasbon-Frodl E, Koehler U, Keller G, Vogelsang H, Dietmaier W, Kopp R, Siebers U, Schmitt W, Neitzel B, Gruber M, Doerner C, Kerker B, Ruemmele P, Henke G, Holinski-Feder E (2005) Deletions account for 17% of pathogenic germline alterations in MLH1 and MSH2 in hereditary nonpolyposis colorectal cancer (HNPCC) families. Genet Test 9(2):138–146. https://doi.org/10.1089/gte.2005.9.138
Mangold E, Pagenstecher C, Friedl W, Mathiak M, Buettner R, Engel C, Loeffler M, Holinski-Feder E, Muller-Koch Y, Keller G, Schackert HK, Kruger S, Goecke T, Moeslein G, Kloor M, Gebert J, Kunstmann E, Schulmann K, Ruschoff J, Propping P (2005) Spectrum and frequencies of mutations in MSH2 and MLH1 identified in 1721 German families suspected of hereditary nonpolyposis colorectal cancer. Int J Cancer 116(5):692–702. https://doi.org/10.1002/ijc.20863
Morak M, Ibisler A, Keller G, Jessen E, Laner A, Gonzales-Fassrainer D, Locher M, Massdorf T, Nissen AM, Benet-Pages A, Holinski-Feder E (2018) Comprehensive analysis of the MLH1 promoter region in 480 patients with colorectal cancer and 1150 controls reveals new variants including one with a heritable constitutional MLH1 epimutation. J Med Genet 55(4):240–248. https://doi.org/10.1136/jmedgenet-2017-104744
Thompson BA, Spurdle AB, Plazzer JP, Greenblatt MS, Akagi K, Al-Mulla F, Bapat B, Bernstein I, Capella G, den Dunnen JT, du Sart D, Fabre A, Farrell MP, Farrington SM, Frayling IM, Frebourg T, Goldgar DE, Heinen CD, Holinski-Feder E, Kohonen-Corish M, Robinson KL, Leung SY, Martins A, Moller P, Morak M, Nystrom M, Peltomaki P, Pineda M, Qi M, Ramesar R, Rasmussen LJ, Royer-Pokora B, Scott RJ, Sijmons R, Tavtigian SV, Tops CM, Weber T, Wijnen J, Woods MO, Macrae F, Genuardi M (2014) Application of a 5-tiered scheme for standardized classification of 2,360 unique mismatch repair gene variants in the InSiGHT locus-specific database. Nat Genet 46(2):107–115. https://doi.org/10.1038/ng.2854
Plazzer JP, Sijmons RH, Woods MO, Peltomaki P, Thompson B, Den Dunnen JT, Macrae F (2013) The InSiGHT database: utilizing 100 years of insights into Lynch syndrome. Fam Cancer 12(2):175–180. https://doi.org/10.1007/s10689-013-9616-0
Borras E, Pineda M, Blanco I, Jewett EM, Wang F, Teule A, Caldes T, Urioste M, Martinez-Bouzas C, Brunet J, Balmana J, Torres A, Ramon y Cajal T, Sanz J, Perez-Cabornero L, Castellvi-Bel S, Alonso A, Lanas A, Gonzalez S, Moreno V, Gruber SB, Rosenberg NA, Mukherjee B, Lazaro C, Capella G (2010) MLH1 founder mutations with moderate penetrance in Spanish Lynch syndrome families. Cancer Res 70(19):7379–7391. https://doi.org/10.1158/0008-5472.CAN-10-0570
Haraldsdottir S, Rafnar T, Frankel WL, Einarsdottir S, Sigurdsson A, Hampel H, Snaebjornsson P, Masson G, Weng D, Arngrimsson R, Kehr B, Yilmaz A, Haraldsson S, Sulem P, Stefansson T, Shields PG, Sigurdsson F, Bekaii-Saab T, Moller PH, Steinarsdottir M, Alexiusdottir K, Hitchins M, Pritchard CC, de la Chapelle A, Jonasson JG, Goldberg RM, Stefansson K (2017) Comprehensive population-wide analysis of Lynch syndrome in Iceland reveals founder mutations in MSH6 and PMS2. Nat Commun 8:14755. https://doi.org/10.1038/ncomms14755
Lynch HT, Coronel SM, Okimoto R, Hampel H, Sweet K, Lynch JF, Barrows A, Wijnen J, van der Klift H, Franken P, Wagner A, Fodde R, de la Chapelle A (2004) A founder mutation of the MSH2 gene and hereditary nonpolyposis colorectal cancer in the United States. JAMA 291(6):718–724. https://doi.org/10.1001/jama.291.6.718
Tomsic J, Liyanarachchi S, Hampel H, Morak M, Thomas BC, Raymond VM, Chittenden A, Schackert HK, Gruber SB, Syngal S, Viel A, Holinski-Feder E, Thibodeau SN, de la Chapelle A (2012) An American founder mutation in MLH1. Int J Cancer 130(9):2088–2095. https://doi.org/10.1002/ijc.26233
von Salome J, Liu T, Keihas M, Morak M, Holinski-Feder E, Berry IR, Moilanen JS, Baert-Desurmont S, Lindblom A, Lagerstedt-Robinson K (2018) Haplotype analysis suggest that the MLH1 c.2059C%3eT mutation is a Swedish founder mutation. Fam Cancer 17(4):531–537. https://doi.org/10.1007/s10689-017-0067-x
Di Resta C, Galbiati S, Carrera P, Ferrari M (2018) Next-generation sequencing approach for the diagnosis of human diseases: open challenges and new opportunities. EJIFCC 29(1):4–14
Kempers MJ, Kuiper RP, Ockeloen CW, Chappuis PO, Hutter P, Rahner N, Schackert HK, Steinke V, Holinski-Feder E, Morak M, Kloor M, Buttner R, Verwiel ET, van Krieken JH, Nagtegaal ID, Goossens M, van der Post RS, Niessen RC, Sijmons RH, Kluijt I, Hogervorst FB, Leter EM, Gille JJ, Aalfs CM, Redeker EJ, Hes FJ, Tops CM, van Nesselrooij BP, van Gijn ME, Gomez Garcia EB, Eccles DM, Bunyan DJ, Syngal S, Stoffel EM, Culver JO, Palomares MR, Graham T, Velsher L, Papp J, Olah E, Chan TL, Leung SY, van Kessel AG, Kiemeney LA, Hoogerbrugge N, Ligtenberg MJ (2011) Risk of colorectal and endometrial cancers in EPCAM deletion-positive Lynch syndrome: a cohort study. Lancet Oncol 12(1):49–55. https://doi.org/10.1016/S1470-2045(10)70265-5
Kuiper RP, Vissers LE, Venkatachalam R, Bodmer D, Hoenselaar E, Goossens M, Haufe A, Kamping E, Niessen RC, Hogervorst FB, Gille JJ, Redeker B, Tops CM, van Gijn ME, van den Ouweland AM, Rahner N, Steinke V, Kahl P, Holinski-Feder E, Morak M, Kloor M, Stemmler S, Betz B, Hutter P, Bunyan DJ, Syngal S, Culver JO, Graham T, Chan TL, Nagtegaal ID, van Krieken JH, Schackert HK, Hoogerbrugge N, van Kessel AG, Ligtenberg MJ (2011) Recurrence and variability of germline EPCAM deletions in Lynch syndrome. Hum Mutat 32(4):407–414. https://doi.org/10.1002/humu.21446
Arnold AM, Morak M, Benet-Pagès A, Laner A, Frishman D, Holinski-Feder E (2019) Targeted deep intronic sequencing in a cohort of unexplained cases of suspected Lynch Syndrome. Eur J Hum Genet. https://doi.org/10.1038/s41431-019-0536-9
Rhees J, Arnold M, Boland CR (2014) Inversion of exons 1–7 of the MSH2 gene is a frequent cause of unexplained Lynch syndrome in one local population. Fam Cancer 13(2):219–225. https://doi.org/10.1007/s10689-013-9688-x
Wagner A, van der Klift H, Franken P, Wijnen J, Breukel C, Bezrookove V, Smits R, Kinarsky Y, Barrows A, Franklin B, Lynch J, Lynch H, Fodde R (2002) A 10-Mb paracentric inversion of chromosome arm 2p inactivates MSH2 and is responsible for hereditary nonpolyposis colorectal cancer in a North-American kindred. Genes Chromosomes Cancer 35(1):49–57. https://doi.org/10.1002/gcc.10094
Chen JM (2008) The 10-Mb paracentric inversion of chromosome arm 2p in activating MSH2 and causing hereditary nonpolyposis colorectal cancer: re-annotation and mutational mechanisms. Genes Chromosomes Cancer 47(6):543–545. https://doi.org/10.1002/gcc.20556
Liu Q, Hesson LB, Nunez AC, Packham D, Williams R, Ward RL, Sloane MA (2016) A cryptic paracentric inversion of MSH2 exons 2–6 causes Lynch syndrome. Carcinogenesis 37(1):10–17. https://doi.org/10.1093/carcin/bgv154
Morak M, Koehler U, Schackert HK, Steinke V, Royer-Pokora B, Schulmann K, Kloor M, Hochter W, Weingart J, Keiling C, Massdorf T, Holinski-Feder E, Hc G (2011) Biallelic MLH1 SNP cDNA expression or constitutional promoter methylation can hide genomic rearrangements causing Lynch syndrome. J Med Genet 48(8):513–519. https://doi.org/10.1136/jmedgenet-2011-100050
Morak M, Schaefer K, Steinke-Lange V, Koehler U, Keinath S, Massdorf T, Mauracher B, Rahner N, Bailey J, Kling C, Haeusser T, Laner A, Holinski-Feder E (2019) Full-length transcript amplification and sequencing as universal method to test mRNA integrity and biallelic expression in mismatch repair genes. Eur J Hum Genet. https://doi.org/10.1038/s41431-019-0472-8
van der Klift HM, Tops CM, Hes FJ, Devilee P, Wijnen JT (2012) Insertion of an SVA element, a nonautonomous retrotransposon, in PMS2 intron 7 as a novel cause of Lynch syndrome. Hum Mutat 33(7):1051–1055. https://doi.org/10.1002/humu.22092
Tattini L, D'Aurizio R, Magi A (2015) Detection of genomic structural variants from next-generation sequencing data. Front Bioeng Biotechnol 3:92. https://doi.org/10.3389/fbioe.2015.00092
Don RH, Cox PT, Wainwright BJ, Baker K, Mattick JS (1991) 'Touchdown' PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res 19(14):4008
Clendenning M, Hampel H, LaJeunesse J, Lindblom A, Lockman J, Nilbert M, Senter L, Sotamaa K, de la Chapelle A (2006) Long-range PCR facilitates the identification of PMS2-specific mutations. Hum Mutat 27(5):490–495. https://doi.org/10.1002/humu.20318
Dominguez-Valentin M, Nakken S, Tubeuf H, Vodak D, Ekstrom PO, Nissen AM, Morak M, Holinski-Feder E, Martins A, Moller P, Hovig E (2018) Identification of genetic variants for clinical management of familial colorectal tumors. BMC Med Genet 19(1):26. https://doi.org/10.1186/s12881-018-0533-9
Morak M, Heidenreich B, Keller G, Hampel H, Laner A, de la Chapelle A, Holinski-Feder E (2014) Biallelic MUTYH mutations can mimic Lynch syndrome. Eur J Hum Genet 22(11):1334–1337. https://doi.org/10.1038/ejhg.2014.15
Palles C, Cazier JB, Howarth KM, Domingo E, Jones AM, Broderick P, Kemp Z, Spain SL, Guarino E, Salguero I, Sherborne A, Chubb D, Carvajal-Carmona LG, Ma Y, Kaur K, Dobbins S, Barclay E, Gorman M, Martin L, Kovac MB, Humphray S, Consortium C, Consortium WGS, Lucassen A, Holmes CC, Bentley D, Donnelly P, Taylor J, Petridis C, Roylance R, Sawyer EJ, Kerr DJ, Clark S, Grimes J, Kearsey SE, Thomas HJ, McVean G, Houlston RS, Tomlinson I (2013) Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat Genet 45(2):136–144. https://doi.org/10.1038/ng.2503
Valle L, Hernandez-Illan E, Bellido F, Aiza G, Castillejo A, Castillejo MI, Navarro M, Segui N, Vargas G, Guarinos C, Juarez M, Sanjuan X, Iglesias S, Alenda C, Egoavil C, Segura A, Juan MJ, Rodriguez-Soler M, Brunet J, Gonzalez S, Jover R, Lazaro C, Capella G, Pineda M, Soto JL, Blanco I (2014) New insights into POLE and POLD1 germline mutations in familial colorectal cancer and polyposis. Hum Mol Genet 23(13):3506–3512. https://doi.org/10.1093/hmg/ddu058
Clendenning M, Buchanan DD, Walsh MD, Nagler B, Rosty C, Thompson B, Spurdle AB, Hopper JL, Jenkins MA, Young JP (2011) Mutation deep within an intron of MSH2 causes Lynch syndrome. Fam Cancer 10(2):297–301. https://doi.org/10.1007/s10689-011-9427-0
Acknowledgements
We thank the Deutsche Krebshilfe e.V. and the Wilhelm Sander-Stiftung for funding this work. We also thank the medical doctors and patients for their participation in this study, as well as the ERN GENTURIS and the German Consortium for Familial Intestinal Cancer for their support. Furthermore, we appreciate Professor Robyn L. Ward and Dr. Mathew A. Sloane from the University of New South Wales, Sydney, Australia and Dr. Jennifer Rhees from the Gastrointestinal Cancer Research Laboratory, Baylor University Medical Center, Dallas, TX, USA for generously providing us a positive control DNA sample for a MSH2 inversion each.
Funding
This study was funded by the Deutsche Krebshilfe e.V. grant number 80935055 and the Wilhelm Sander-Stiftung Grant Number 80735127.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10689_2020_159_MOESM1_ESM.tif
Schematic presentation of the PCR-based detection method used as a screening for rearrangements, with one example each for an inversion (a) and an insertion (b). In the wildtype allele above, the primers of the control PCR are depicted as arrows in green and yellow. Here, the breakpoint(s) of the inversion or insertion are marked with red flashes within the wildtype allele, and the allele with the rearrangement is shown below. For the newly generated breakpoints, rearrangement-specific PCR amplifications were set up. (a) The inversion-specific fusion breakpoints are detected by PCR amplifications, one using the green primer pair, and the other using the yellow primer pair in the rearrangement allele. (b) The insertion of the 2.2 kb SVA element in PMS2 intron 7 is detected by amplification of an insertion-specific PCR product using primer combination in green and red. (TIF 831 kb)
Rights and permissions
About this article
Cite this article
Morak, M., Steinke-Lange, V., Massdorf, T. et al. Prevalence of CNV-neutral structural genomic rearrangements in MLH1, MSH2, and PMS2 not detectable in routine NGS diagnostics. Familial Cancer 19, 161–167 (2020). https://doi.org/10.1007/s10689-020-00159-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10689-020-00159-4