Abstract
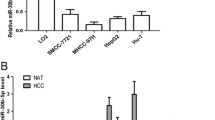
MiR-23a-3p has been shown to promote liver cancer cell growth and metastasis and regulate that of chemosensitivity. Protocadherin17 (PCDH17) is a tumor suppressor gene and plays an essential part in cell cycle of hepatocellular carcinoma (HCC). This study aimed at evaluating the effects of miR-23a-3p and PCDH17 on HCC cell cycle and underlining the mechanism. The level of miR-23a-3p was up-regulated, while PCDH17 level was down-regulated in HCC tissues compared to adjacent tissues. For the in vitro studies, high expression of miR-23a-3p down-regulated PCDH17 level; increased cell viability; promoted G1/S cell cycle transition; up-regulated cyclin D1, cyclin E, CDK2, CDK4, p-p27, and p-RB levels; and down-regulated the expression of p27. Dual luciferase reporter assay suggested PCDH17 was a target gene of miR-23a-3p. MiR-23a-3p inhibitor and PCDH17 siRNA led to an increase in cell viability and the number of cells in the S phase and up-regulated cyclin D1 and cyclin E levels, compared with miR-23a-3p inhibitor and NC siRNA group. For the in vivo experiments, high expression of miR-23a-3p promoted tumor growth and reduced PCDH17 level in the cytoplasm. These results indicated that high expression of miR-23a-3p might promote G1/S cell cycle transition by targeting PCDH17 in HCC cells. The miR-23a-3p could be considered as a molecular target for HCC detection.





Similar content being viewed by others
References
Agostini S, Mancuso R, Liuzzo G, Bolognesi E, Costa AS, Bianchi A, Clerici M (2019) Serum miRNAs expression and SNAP-25 genotype in Alzheimer's disease. Front Aging Neurosci 11:52. https://doi.org/10.3389/fnagi.2019.00052
Bao L, Zhao J, Dai X, Wang Y, Ma R, Su Y, Cui H, Niu J, Bai S, Xiao Z, Yuan H, Yang Z, Li C, Cheng R, Ren X (2014) Correlation between miR-23a and onset of hepatocellular carcinoma. Clin Res Hepatol Gastroenterol 38:318–330. https://doi.org/10.1016/j.clinre.2013.12.002
Dang Z, Shangguan J, Zhang C, Hu P, Ren Y, Lv Z, Xiang H, Wang X (2016) Loss of protocadherin-17 (PCDH-17) promotes metastasis and invasion through hyperactivation of EGFR/MEK/ERK signaling pathway in hepatocellular carcinoma. Tumour Biol 37:2527–2535. https://doi.org/10.1007/s13277-015-3970-5
Diehl JA (2002) Cycling to cancer with cyclin D1. Cancer Biol Ther 1:226–231. https://doi.org/10.4161/cbt.72
Eissa S, Matboli M, Shehata HH (2015) Breast tissue-based microRNA panel highlights microRNA-23a and selected target genes as putative biomarkers for breast cancer. Transl Res 165:417–427. https://doi.org/10.1016/j.trsl.2014.10.001
He Y, Wang Z, Liu C, Gong Z, Li Y, Lu T, Hu G (2019) Protocadherin 17 is a tumor suppressor and is frequently methylated in nasopharyngeal carcinoma. Cancer Manag Res 11:1601–1613. https://doi.org/10.2147/cmar.s191102
Hu X, Sui X, Li L, Huang X, Rong R, Su X, Shi Q, Mo L, Shu X, Kuang Y, Tao Q, He C (2013) Protocadherin 17 acts as a tumour suppressor inducing tumour cell apoptosis and autophagy, and is frequently methylated in gastric and colorectal cancers. J Pathol 229:62–73. https://doi.org/10.1002/path.4093
Huang S, He X, Ding J, Liang L, Zhao Y, Zhang Z, Yao X, Pan Z, Zhang P, Li J, Wan D, Gu J (2008) Upregulation of miR-23a approximately 27a approximately 24 decreases transforming growth factor-β-induced tumor-suppressive activities in human hepatocellular carcinoma cells. Int J Cancer 123:972–978. https://doi.org/10.1002/ijc.23580
Huang H, Liu Y, Yu P, Qu J, Guo Y, Li W, Wang S, Zhang J (2018) MiR-23a transcriptional activated by Runx2 increases metastatic potential of mouse hepatoma cell via directly targeting Mgat3. Sci Rep 8:7366. https://doi.org/10.1038/s41598-018-25768-z
Hydbring P, Castell A, Larsson LG (2017) MYC modulation around the CDK2/p27/SKP2 axis. Genes 8. https://doi.org/10.3390/genes8070174
Jin AH, Wei ZL (2015) Molecular mechanism of increased sensitivity of cisplatin to ovarian cancer by inhibition of microRNA-23a expression. Int J Clin Exp Med 8:13329–13334
Kanska J, Zakhour M, Taylor-Harding B, Karlan BY, Wiedemeyer WR (2016) Cyclin E as a potential therapeutic target in high grade serous ovarian cancer. Gynecol Oncol 143:152–158. https://doi.org/10.1016/j.ygyno.2016.07.111
Kim SY, Yasuda S, Tanaka H, Yamagata K, Kim H (2011) Non-clustered protocadherin. Cell Adhes Migr 5:97–105. https://doi.org/10.4161/cam.5.2.14374
Krepelkova I, Mrackova T, Izakova J, Dvorakova B, Chalupova L, Mikulik R, Slaby O, Bartos M, Ruzicka V (2019) Evaluation of miRNA detection methods for the analytical characteristic necessary for clinical utilization. BioTechniques 66:277–284. https://doi.org/10.2144/btn-2019-0021
Kutay H, Bai S, Datta J, Motiwala T, Pogribny I, Frankel W, Jacob ST, Ghoshal K (2006) Downregulation of miR-122 in the rodent and human hepatocellular carcinomas. J Cell Biochem 99:671–678. https://doi.org/10.1002/jcb.20982
Larrea MD, Liang J, Da Silva T, Hong F, Shao SH, Han K, Dumont D, Slingerland JM (2008) Phosphorylation of p27Kip1 regulates assembly and activation of cyclin D1-Cdk4. Mol Cell Biol 28:6462–6472. https://doi.org/10.1128/mcb.02300-07
Latchana N, Abrams ZB, Howard JH, Regan K, Jacob N, Fadda P, Terando A, Markowitz J, Agnese D, Payne P, Carson WE (2017) Plasma microRNA levels following resection of metastatic melanoma. Bioinf Biol Insights 11:1177932217694837. https://doi.org/10.1177/1177932217694837
Lian S, Shi R, Bai T, Liu Y, Miao W, Wang H, Liu X, Fan Y (2013) Anti-miRNA-23a oligonucleotide suppresses glioma cells growth by targeting apoptotic protease activating factor-1. Curr Pharm Des 19:6382–6389. https://doi.org/10.2174/13816128113199990509
Luo M, Sun G, Sun JW (2019) MiR-196b affects the progression and prognosis of human LSCC through targeting PCDH-17. Auris Nasus Larynx 46:583–592. https://doi.org/10.1016/j.anl.2018.10.020
Mens MMJ, Ghanbari M (2018) Cell cycle regulation of stem cells by microRNAs. Stem Cell Rev 14:309–322. https://doi.org/10.1007/s12015-018-9808-y
Mohamed AA, Ali-Eldin ZA, Elbedewy TA, El-Serafy M, Ali-Eldin FA, AbdelAziz H (2017) MicroRNAs and clinical implications in hepatocellular carcinoma. World J Hepatol 9:1001–1007. https://doi.org/10.4254/wjh.v9.i23.1001
Morishita A, Masaki T (2015) miRNA in hepatocellular carcinoma. Hepatol Res 45:128–141. https://doi.org/10.1111/hepr.12386
Quan J, Pan X, Li Y, Hu Y, Tao L, Li Z, Zhao L, Wang J, Li H, Lai Y, Zhou L, Lin C, Gui Y, Ye J, Zhang F, Lai Y (2019) MiR-23a-3p acts as an oncogene and potential prognostic biomarker by targeting PNRC2 in RCC. Biomed Pharmacother 110:656–666. https://doi.org/10.1016/j.biopha.2018.11.065
Salloum-Asfar S, Teruel-Montoya R, Arroyo AB, Garcia-Barbera N, Chaudhry A, Schuetz E, Luengo-Gil G, Vicente V, Gonzalez-Conejero R, Martinez C (2014) Regulation of coagulation factor XI expression by microRNAs in the human liver. PLoS One 9:e111713. https://doi.org/10.1371/journal.pone.0111713
Wang N, Zhu M, Tsao SW, Man K, Zhang Z, Feng Y (2013) MiR-23a-mediated inhibition of topoisomerase 1 expression potentiates cell response to etoposide in human hepatocellular carcinoma. Mol Cancer 12:119. https://doi.org/10.1186/1476-4598-12-119
Wang XB, Lin YL, Li ZG, Ma JH, Li J, Ma JG (2014) Protocadherin 17 promoter methylation in tumour tissue from patients with bladder transitional cell carcinoma. J Int Med Res 42:292–299. https://doi.org/10.1177/0300060513504364
Wang X, Lu J, Cao J, Ma B, Gao C, Qi F (2018) MicroRNA-18a promotes hepatocellular carcinoma proliferation, migration, and invasion by targeting Bcl2L10. OncoTargets Ther 11:7919–7934. https://doi.org/10.2147/ott.s180971
Wee P, Wang Z (2017) Cell cycle synchronization of HeLa cells to assay EGFR pathway activation. Methods Mol Biol 1652:167–181. https://doi.org/10.1007/978-1-4939-7219-7_13
Wikman H, Kettunen E (2006) Regulation of the G1/S phase of the cell cycle and alterations in the RB pathway in human lung cancer. Expert Rev Anticancer Ther 6:515–530. https://doi.org/10.1586/14737140.6.4.515
Wu CW, Storey KB (2018) Regulation of Smad mediated microRNA transcriptional response in ground squirrels during hibernation. Mol Cell Biochem 439:151–161. https://doi.org/10.1007/s11010-017-3144-4
Xu F, Li Q, Wang Z, Cao X (2019) Sinomenine inhibits proliferation, migration, invasion and promotes apoptosis of prostate cancer cells by regulation of miR-23a. Biomed Pharmacother 112:108592. https://doi.org/10.1016/j.biopha.2019.01.053
Xu ZJ, Ma JC, Zhou JD, Wen XM, Yao DM, Zhang W, Ji RB, Wu DH, Tang LJ, Deng ZQ, Qian J, Lin J (2019) Reduced protocadherin17 expression in leukemia stem cells: the clinical and biological effect in acute myeloid leukemia. J Transl Med 17:102. https://doi.org/10.1186/s12967-019-1851-1
Yan Y, Liang Z, Du Q, Yang M, Geller DA (2016) MicroRNA-23a downregulates the expression of interferon regulatory factor-1 in hepatocellular carcinoma cells. Oncol Rep 36:633–640. https://doi.org/10.3892/or.2016.4864
Yang Y, Liu J, Li X, Li JC (2012) PCDH17 gene promoter demethylation and cell cycle arrest by genistein in gastric cancer. Histol Histopathol 27:217–224. https://doi.org/10.14670/hh-27.217
Yin X, Xiang T, Mu J, Mao H, Li L, Huang X, Li C, Feng Y, Luo X, Wei Y, Peng W, Ren G, Tao Q (2016) Protocadherin 17 functions as a tumor suppressor suppressing Wnt/β-catenin signaling and cell metastasis and is frequently methylated in breast cancer. Oncotarget 7:51720–51732. https://doi.org/10.18632/oncotarget.10102
Funding
This study was funded by a grant from the Special Project for Health Care of Jilin Province Provincial Department of Finance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All animal experimental protocols were reviewed and approved by the Ethics Committee of Jilin University for the use of laboratory animals.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• High expression of miR-23a-3p promoted G1/S cell cycle transition.
• Dual luciferase reporter assay suggested PCDH17 was a target gene of miR-23a-3p.
• MiR-23a-3p inhibitor and PCDH17 siRNA up-regulated cyclin D1 and cyclin E levels.
• High expression of miR-23a-3p promoted tumor growth and reduced PCDH17 level
Electronic supplementary material
Fig. S1
Effect of miR-23a-3p on Hep3B and HepG2 cells. After transfection for 48 h, the miR-23a-3p level of Hep3B (a) and HepG2 (b) cells was detected with RT-qPCR. Results were present as mean ± SD (n = 3). ***P < 0.001 vs. the NC mimics group; ###P < 0.001 vs. the NC inhibitor group (PNG 20 kb)
Fig. S2
Effect of PCDH17 on Hep3B and HepG2 cells. After transfection for 48 h, the expression of PCDH17 was detected in Hep3B (a) and HepG2 (b) cells with western blotting. β-actin was used as internal reference. Results were present as mean ± SD (n = 3). *P < 0.05 and **P < 0.01 vs. the NC siRNA groups; ###P < 0.001 vs. the empty vector groups (PNG 84 kb)
Fig. S3
Effect of PCDH17 on cell cycle of HCC cells. a Cell viability was detected by CCK-8 assay. b After transfection for 48 h, cell cycle was evaluated with flow cytometry. Results were present as mean ± SD (n = 3). *P < 0.05 and **P < 0.01 vs. the NC siRNA group; #P < 0.05 and ##P < 0.01 vs. the empty vector group (PNG 158 kb)
Rights and permissions
About this article
Cite this article
Xiang, Y., Yang, Y., Lin, C. et al. MiR-23a-3p promoted G1/S cell cycle transition by targeting protocadherin17 in hepatocellular carcinoma. J Physiol Biochem 76, 123–134 (2020). https://doi.org/10.1007/s13105-020-00726-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-020-00726-4