Abstract
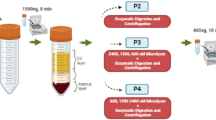
Effective harvesting procedure for adipose tissue is demanded by the affordable Good Manufacturing Practice-Compliant Production of clinical-grade adipose tissue-derived stem cells (hADSCs). Enzymatic digestion using collagenase is the most reliable method of adipose tissue-derived stem cells (hADSCs) isolation, while the optimized loading volume ratios of digestion to container during the shaking process of adipose tissue and collagenase mixture are still lacking. This study was conducted to determine the optimized loading volume ratio (mixture to container) for enzymatic digestion of Stromal/Stem Cells from lipoaspirate. Lipoaspirates were obtained from twelve women immediately after liposuction. Then tissue from each patient was divided into four groups according to different loading volume ratios in 50 ml centrifugal tube: 0.2 group, 0.4 group, 0.6 group, 0.8 group. Stromal vascular fractions (SVF) were obtained from each group, then total cell counts, viability and viable cell count were performed. hADSCs were harvested at passage (P) 2, whose morphologies, immunophenotypes, proliferation, and tri-differentiation abilities were compared. 0.4 loading volume ratio provided the highest cell yield, favorable viability and viable cell yield. The proliferation and triple differentiation ability of hADSCs obtained by 0.4 group was not inferior to that of other groups. Therefore, 0.4 may be the optimal loading volume ratio for hADSCs isolation from lipoaspirate by enzymatic digestion in current setting.






Similar content being viewed by others
References
Alexandra CG, Rodriguez RL, Sheri S, Singh DP, Goldberg NH, John ML (2014) Comparison between stromal vascular cells’ isolation with enzymatic digestion and mechanical processing of aspirated adipose tissue. Plast Reconstr Surg 134:54
Alstrup T, Eijken M, Bohn AB, Moller B, Damsgaard TE (2019) Isolation of adipose tissue-derived stem cells: enzymatic digestion in combination with mechanical distortion to increase adipose tissue-derived stem cell yield from human aspirated fat. Curr Protoc Stem Cell Biol 48:e68. https://doi.org/10.1002/cpsc.68
Aluigi MG et al (2009) Corrigendum to pre-adipocytes commitment to neurogenesis 1: preliminary localisation of cholinergic molecules. Cell Biol Int 33:594–601
Aronowitz JA, Lockhart RA, Hakakian CS (2015) Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus 4:1–9
Aronowitz JA, Lockhart RA, Hakakian CS, Birnbaum ZE (2016) Adipose stromal vascular fraction isolation: a head-to-head comparison of 4 cell separation systems #2. Ann Plast Surg 77:354–362
Aust L et al (2004) Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy 6:7–14
Bahrami B, Hosseini A, Talei AR, Ghaderi A, Razmkhah M (2017) Adipose derived stem cells exert immunomodulatory effects on natural killer cells in breast cancer. Cell J 19:137–145
Baptista LS, Amaral RJFC, Carias RBV, Marcelo A, Cesar CDS, Radovan B (2009) An alternative method for the isolation of mesenchymal stromal cells derived from lipoaspirate samples. Cytotherapy 11:706–715
Bourin P et al (2013) Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 15:641–648. https://doi.org/10.1016/j.jcyt.2013.02.006
Cheng KH, Kuo TL, Kuo KK, Hsiao CC (2011) Human adipose-derived stem cells: isolation, characterization and current application in regeneration medicine. Genomic Med Biomarkers Health Sci 3:53–62
Chung MT et al (2013) CD90 (Thy-1)-positive selection enhances osteogenic capacity of human adipose-derived stromal cells. Tissue Eng Part A 19:989–997. https://doi.org/10.1089/ten.TEA.2012.0370
Condé-Green A, Kotamarti VS, Sherman LS, Keith JD, Lee ES, Granick MS, Rameshwar P (2016) Shift toward mechanical isolation of adipose-derived stromal vascular fraction: review of upcoming techniques. Plast Reconstr Surg Global Open 4:e1017
Dejesus MJ (2004) TubeSpin satellites: a fast track approach for process development with animal cells using shaking technology. Biochem Eng J 17(3):217–223
Domenis R et al (2015) Adipose tissue derived stem cells: in vitro and in vivo analysis of a standard and three commercially available cell-assisted lipotransfer techniques. Stem Cell Res Ther 6:1–15
Edoardo R, Giorgia C, Sabrina B, Guido L (2014) A novel and effective strategy for the isolation of adipose-derived stem cells: minimally manipulated adipose-derived stem cells for more rapid and safe stem cell therapy. Plast Reconstr Surg 133:1406–1409
Faustini M et al (2010) Nonexpanded mesenchymal stem cells for regenerative medicine: yield in stromal vascular fraction from adipose tissues. Tissue Eng Part C Methods 16:1515
Fraser JK, Hicok KC, Shanahan R, Zhu M, Miller S, Arm DM (2014) The Celution® system: automated processing of adipose-derived regenerative cells in a functionally closed system. Adv Wound Care 3(1):38–45
Hassan WU, Greiser U, Wang W (2014) Role of adipose-derived stem cells in wound healing. Wound Repair Regen 22:313–325
Jonathan D, Chang WT, Dragoo JL (2018) The use of vibrational energy to isolate adipose-derived stem cells. Plast Reconstr Surg Global Open 6:1
Kim YS, Kwon OR, Choi YJ, Suh DS, Heo DB, Koh YG (2015) Comparative matched-pair analysis of the injection versus implantation of mesenchymal stem cells for knee osteoarthritis. Am J Sports Med 43:2738–2746
Koh YG, Choi YJ, Kwon OR, Kim YS (2014) Second-look arthroscopic evaluation of cartilage lesions after mesenchymal stem cell implantation in osteoarthritic knees. Am J Sports Med 42:176–185
Koh YG, Kwon OR, Kim YS, Choi YJ, Tak DH (2016) Adipose-derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial. Arthroscopy 32(1):97–109
Kotaro Y et al (2010) Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol 208:64–76
Kuo YR, Wang CT, Cheng JT, Kao GS, Chiang YC, Wang CJ (2016) Adipose-derived stem cells accelerate diabetic wound healing through the induction of autocrine and paracrine effects. Cell Transplant 25:71–81
Lin K et al (2008) Characterization of adipose tissue-derived cells isolated with the celution system. Cytotherapy 10:417–426
Makarov AV, Arutyunyan IV, Bol Shakova GB, Volkov AV, Gol Dshtein DV (2009) Morphological changes in paraurethral area after introduction of tissue engineering construct on the basis of adipose tissue stromal cells. Bull Exp Biol Med 148:719–724
Markarian CF et al (2014) Isolation of adipose-derived stem cells: a comparison among different methods. Biotech Lett 36:693–702
Meier K, Klöckner W, Bonhage B, Antonov E, Regestein L, Büchs J (2016) Correlation for the maximum oxygen transfer capacity in shake flasks for a wide range of operating conditions and for different culture media. Biochem Eng J 109:228–235
Mitchell JB et al (2010) Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells 24:376–385
Mizuno H, Tobita M, Uysal AC (2012) Concise review: adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells 30:804–810. https://doi.org/10.1002/stem.1076
Oberbauer E, Steffenhagen C, Wurzer C, Gabriel C, Redl H, Wolbank S (2015) Enzymatic and non-enzymatic isolation systems for adipose tissue-derived cells: current state of the art. Cell Regen 4:7. https://doi.org/10.1186/s13619-015-0020-0
Oedayrajsingh-Varma MJ et al (2006) Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy 8:166–177
Pittenger MF et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Raposio E, Simonacci F, Perrotta RE (2017) Adipose-derived stem cells: comparison between two methods of isolation for clinical applications. Annals Med Surg 20:87
Scanarotti C et al (2010) Neurogenic-committed human pre-adipocytes express CYP1A isoforms. Chem Biol Interact 184:474–483
Schiefelbein S, Frohlich A, John GT, Beutler F, Wittmann C, Becker J (2013) Oxygen supply in disposable shake-flasks: prediction of oxygen transfer rate, oxygen saturation and maximum cell concentration during aerobic growth. Biotechnol Lett 35:1223–1230. https://doi.org/10.1007/s10529-013-1203-9
Shah FS, Wu X, Dietrich M, Rood J, Gimble JM (2013) A non-enzymatic method for isolating human adipose tissue-derived stromal stem cells. Cytotherapy 15:979–985. https://doi.org/10.1016/j.jcyt.2013.04.001
Suga H et al (2010) Adipose tissue remodeling under ischemia: death of adipocytes and activation of stem/progenitor cells. Plast Reconstr Surg 126:1911
Sun AJ, Qiao L, Huang C, Zhang X, Li YQ, Yang XQ (2018) Comparison of mouse brown and white adiposederived stem cell differentiation into pacemakerlike cells induced by TBX18 transduction. Mol med rep 17:7055–7064
Suresh S, Srivastava VC, Mishra IM (2009) Critical analysis of engineering aspects of shaken flask bioreactors. Crit Rev Biotechnol 29:255–278. https://doi.org/10.3109/07388550903062314
Tevlin R, McArdle A, Brett E, Chung MT, Longaker MT (2016) A novel method of human adipose-derived stem cell isolation with resultant increased cell yield. Plast Reconstr Surg 138:983e–996e
Trivisonno A, Di RG, Cannistra C, Finocchi V, Torres FS, Monti M, Toietta G (2014) Harvest of superficial layers of fat with a microcannula and isolation of adipose tissue-derived stromal and vascular cells. Aesthetic Surg J 34:601–613
Wankhade UD, Shen M, Kolhe R, Fulzele S (2016) Advances in adipose-derived stem cells isolation, characterization, and application in regenerative tissue engineering. Stem Cells Int. 2016:3206807
Xie K et al (2011a) On-line monitoring of oxygen in Tubespin, a novel, small-scale disposable bioreactor. Cytotechnology 63:345–350. https://doi.org/10.1007/s10616-011-9361-x
Xie Q, Michel PO, Baldi L, Hacker DL, Zhang X, Wurm FM (2011b) TubeSpin bioreactor 50 for the high-density cultivation of Sf-9 insect cells in suspension. Biotechnol Lett 33:897–902. https://doi.org/10.1007/s10529-011-0527-6
Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J (2000) Vascular-specific growth factors and blood vessel formation. Nature 407:242–248. https://doi.org/10.1038/35025215
Yong-Gon K, Seung-Bae J, Oh-Ryong K, Dong-Suk S, Seung-Woo L, Sung-Ho P, Yun-Jin C (2013) Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy J Arthroscopic Related Surg 29:748–755
Zhang X et al (2009) Efficient oxygen transfer by surface aeration in shaken cylindrical containers for mammalian cell cultivation at volumetric scales up to 1000 L. Biochem Eng J 45:41–47. https://doi.org/10.1016/j.bej.2009.02.003
Zhu L et al (2018a) Fluid dynamics of flow fields in a disposable 600-ml orbitally shaken bioreactor. Biochem Eng J 129:84–95
Zhu L, Zhang X, Cheng K, Lv Z, Wang Z (2018b) Characterizing the fluid dynamics of the inverted frusto-conical shaking bioreactor. Biotechnol Prog 34(2):478–485
Zhu LK, Wang H, Song BY, Wang ZL (2018c) Characterizing the fluid dynamics in the flow fields of cylindrical orbitally shaken bioreactors with different geometry sizes. Eng Life Sci 18:570–578
Zuk P et al (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng Part A 7:211–228
Funding
This work was supported by CAMS Innovation Fund for Medical Sciences (CIFMS) (2017-I2M-3-006).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors confirm that this article content has no conflict of interest.
Ethics approval and consent to participate
All human tissue collection was approved by the Ethical Committee of Plastic Surgery Hospital, Chinese Academy of Medical Sciences and Peking Union of Medical College and was carried out in accordance with the approved guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Z., Mu, D., Liu, C. et al. The cell yields and biological characteristics of stromal/stem cells from lipoaspirate with different digestion loading ratio. Cytotechnology 72, 203–215 (2020). https://doi.org/10.1007/s10616-020-00369-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-020-00369-9