Abstract
Background
Children with multidrug-resistant nephrotic syndrome (MRNS) are exposed to drug toxicity (steroids/calcineurin inhibitors (CNI)/mycophenolate mofetil (MMF)) and have an increased risk of kidney disease progression. In small case series, the fully humanized anti-CD20 antibody ofatumumab (OFA) induced remission in children with MRNS when at high dose (10,300 mg/1.73 m2) and partial remission at standard dose (1000 mg/1.73 m2).
Methods
This double-blind randomized placebo-controlled trial tested the efficacy of single infusion OFA in children with proven MRNS and initial chronic renal failure (eGFR [median/range] 119/38–155 ml/min/1.73 m2 in Placebo arm vs. 65/19–103 ml/min/1.73 m2 Intervention). Children who had been resistant to a combination of CNI and steroids, with or without MMF or rituximab, were randomized to receive single infusion OFA (1500 mg/1.73 m2) (Intervention arm) or normal saline (Placebo arm). We assessed complete or partial remission of proteinuria after 3 months (primary outcome), and after 6 and 12 months (secondary outcomes), as well as progression to end-stage kidney disease.
Results
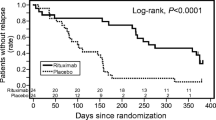
After 13 of the planned 50 children (25%) were randomized, the data safety and monitoring board recommended study termination for futility. All 13 children remained nephrotic. Renal function worsened in 5 children (2 in Intervention arm, 3 in Placebo arm) who required renal replacement therapy during the study period. Circulating CD20 was reduced following OFA infusion and remained low for > 3 months.
Conclusions
OFA given in one single infusion of 1500 mg/1.73 m2 doses does not induce remission in MRNS. Regimens based on higher OFA doses should be tested in clinical trials.
Trial registration
https://clinicaltrials.gov: NCT02394106

Similar content being viewed by others
References
Iijima K, Sako M, Nozu K, Mori R, Tuchida N, Kamei K, Miura K, Aya K, Nakanishi K, Ohtomo Y, Takahashi S, Tanaka R, Kaito H, Nakamura H, Ishikura K, Ito S, Ohashi Y, Rituximab for Childhood-onset Refractory Nephrotic Syndrome (RCRNS) Study Group (2014) Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 384:1273–1281
Ravani P, Bonanni A, Rossi R, Caridi G, Ghiggeri GM (2016) Anti-CD20 antibodies for idiopathic nephrotic syndrome in children. Clin J Am Soc Nephrol 11:710–720
Basu B, Sander A, Roy B, Preussler S, Barua S, Mahapatra TKS, Schaefer F (2018) Efficacy of rituximab vs tacrolimus in pediatric corticosteroid-dependent nephrotic syndrome: a randomized clinical trial. JAMA Pediatr 172:757–764
Magnasco A, Ravani P, Edefonti A, Murer L, Ghio L, Belingheri M, Benetti E, Murtas C, Messina G, Massella L, Porcellini MG, Montagna M, Regazzi M, Scolari F, Ghiggeri GM (2012) Rituximab in children with resistant idiopathic nephrotic syndrome. J Am Soc Nephrol 23:1117–1124
Ravani P, Bonanni A, Ghiggeri GM (2017) Randomised controlled trial comparing ofatumumab to rituximab in children with steroid-dependent and calcineurin inhibitor-dependent idiopathic nephrotic syndrome: study protocol. BMJ Open 7:e013319
Vivarelli M, Colucci M, Bonanni A, Verzani M, Serafinelli J, Emma F, Ghiggeri G (2017) Ofatumumab in two pediatric nephrotic syndrome patients allergic to rituximab. Pediatr Nephrol 32:181–184
Wang CS, Liverman RS, Garro R, George RP, Glumova A, Karp A, Jernigan S, Warshaw B (2017) Ofatumumab for the treatment of childhood nephrotic syndrome. Pediatr Nephrol 32:835–841
Bernard J, Bruel A, Allain-Launay E, Dantal J, Roussey G (2018) Ofatumumab in post-transplantation recurrence of a pediatric steroid-resistant idiopathic nephrotic syndrome. Pediatr Transplant 22:e13175
Basu B (2014) Ofatumumab for rituximab-resistant nephrotic syndrome. N Engl J Med 370:1268–1270
Bonanni A, Rossi R, Murtas C, Ghiggeri GM (2015, 2015) Low-dose ofatumumab for rituximab-resistant nephrotic syndrome. BMJ Case Rep. https://doi.org/10.1136/bcr-2015-210208
Bonanni A, Calatroni M, D’Alessandro M, Signa S, Bertelli E, Cioni M, Di Marco E, Biassoni R, Caridi G, Ingrasciotta G, Bertelli R, Di Donato A, Bruschi M, Canepa A, Piaggio G, Ravani P, Ghiggeri GM (2018) Adverse events linked with the use of chimeric and humanized anti-CD20 antibodies in children with idiopathic nephrotic syndrome. Br J Clin Pharmacol 84:1238–1249
Vincenti F, Ghiggeri GM (2005) New insights into the pathogenesis and the therapy of recurrent focal glomerulosclerosis. Am J Transplant 5:1179–1185
Dello Strologo L, Guzzo I, Laurenzi C, Vivarelli M, Parodi A, Barbano G, Camilla R, Scozzola F, Amore A, Ginevri F, Murer L (2009) Use of rituximab in focal glomerulosclerosis relapses after renal transplantation. Transplantation 88:417–420
Ravani P, Magnasco A, Edefonti A, Murer L, Rossi R, Ghio L, Benetti E, Scozzola F, Pasini A, Dallera N, Sica F, Belingheri M, Scolari F, Ghiggeri GM (2011) Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: a randomized controlled trial. Clin J Am Soc Nephrol 6:1308–1315
Ravani P, Ponticelli A, Siciliano C, Fornoni A, Magnasco A, Sica F, Bodria M, Caridi G, Wei C, Belingheri M, Ghio L, Merscher-Gomez S, Edefonti A, Pasini A, Montini G, Murtas C, Wang X, Muruve D, Vaglio A, Martorana D, Pani A, Scolari F, Reiser J, Ghiggeri GM (2013) Rituximab is a safe and effective long-term treatment for children with steroid and calcineurin inhibitor-dependent idiopathic nephrotic syndrome. Kidney Int 84:1025–1033
Ravani P, Rossi R, Bonanni A, Quinn RR, Sica F, Bodria M, Pasini A, Montini G, Edefonti A, Belingheri M, De Giovanni D, Barbano G, Degl’Innocenti L, Scolari F, Murer L, Reiser J, Fornoni A, Ghiggeri GM (2015) Rituximab in children with steroid-dependent nephrotic syndrome: a multicenter, open-label, noninferiority, randomized controlled trial. J Am Soc Nephrol 26:2259–2266
Funding
The study on ofatumumab was financially supported by the Istituto Giannina Gaslini deriving from “Cinque per mille of IRPEF-Finanziamento della ricerca sanitaria,” the Italian Ministry of Health, The Renal Child Foundation, and the “Fondazione La Nuova Speranza” (“Progetto integrato per la definizione dei meccanismi implicate nella glomerulosclerosifocale”). GC and GMG received a grant from Compagnia San Paolo (ROL-9849).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Before initiation of the study, we obtained written approval of the protocol, the Informed Consent Form, and any information presented to potential subjects from the local Independent Ethics Committee (Comitato Etico Regione Liguria). We also obtained approval from the Italian Drug Agency (Agenzia Italiana del Farmaco, AIFA).
Conflict of interest
The authors declare that they have no conflict of interest
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ravani, P., Pisani, I., Bodria, M. et al. Low-dose ofatumumab for multidrug-resistant nephrotic syndrome in children: a randomized placebo-controlled trial. Pediatr Nephrol 35, 997–1003 (2020). https://doi.org/10.1007/s00467-020-04481-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-020-04481-y