Abstract
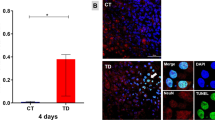
Thiamine deficiency (TD) produces severe neurodegenerative lesions. Studies have suggested that primary neurodegenerative events are associated with both oxidative stress and inflammation. Very little is known about the downstream effects on intracellular signaling pathways involved in neuronal death. The primary aim of this work was to evaluate the modulation of p38MAPK and the expression of heme oxygenase 1 (HO-1) in the central nervous system (CNS). Behavioral, metabolic, and morphological parameters were assessed. Mice were separated into six groups: control (Cont), TD with pyrithiamine (Ptd), TD with pyrithiamine and Trolox (Ptd + Tr), TD with pyrithiamine and dimethyl sulfoxide (Ptd + Dmso), Trolox (Tr) and DMSO (Dmso) control groups and treated for 9 days. Control groups received standard feed (AIN-93M), while TD groups received thiamine deficient feed (AIN-93DT). All the groups were subjected to behavioral tests, and CNS samples were collected for cell viability, histopathology and western blot analyses. The Ptd group showed a reduction in weight gain and feed intake, as well as a reduction in locomotor, grooming, and motor coordination activities. Also, Ptd group showed a robust increase in p38MAPK phosphorylation and mild HO-1 expression in the cerebral cortex and thalamus. The Ptd group showed a decreased cell viability, hemorrhage, spongiosis, and astrocytic swelling in the thalamus. Groups treated with Trolox and DMSO displayed diminished p38MAPK phosphorylation in both the structures, as well as attenuated thalamic lesions and behavioral activities. These data suggest that p38MAPK and HO-1 are involved in the TD-induced neurodegeneration in vivo, possibly modulated by oxidative stress and neuroinflammation.





Similar content being viewed by others
References
Butterworth RF (2009) Thiamine deficiency-related brain dysfunction in chronic liver failure. Metab Brain Dis 24:189–196. https://doi.org/10.1007/s11011-008-9129-y
Haas RH (1988) Thiamin and the brain. Annu Rev Nutr 8:483–515. https://doi.org/10.1146/annurev.nu.08.070188.002411
Vetreno RP, Ramos RL, Anzalone S, Savage LM (2012) Brain and behavioral pathology in an animal model of Wernicke’s encephalopathy and Wernicke-Korsakoff Syndrome. Brain Res 1436:178–192. https://doi.org/10.1016/j.brainres.2011.11.038
Suzuki K, Yamada K, Fukuhara Y, Tsuji A, Shibata K (2017) High-dose thiamine prevents brain lesions and prolongs survival of Slc19a3 -deficient mice. PLoS ONE 12:e0180279. https://doi.org/10.1371/journal.pone.0180279
Apley MD (2015) Consideration of evidence for therapeutic interventions in bovine polioencephalomalacia. Vet Clin North Am 31:151–161. https://doi.org/10.1016/j.cvfa.2014.11.005
Abdou E, Hazell AS (2015) Thiamine deficiency: an update of pathophysiologic mechanisms and future therapeutic considerations. Neurochem Res 40:353–361. https://doi.org/10.1007/s11064-014-1430-z
Butterworth RF, Kril JJ, Harper CG (1993) Thiamine-dependent enzyme changes in the brains of alcoholics: relationship to the Wernicke-Korsakoff syndrome. Alcohol Clin Exp Res 17:1084–1088. https://doi.org/10.1111/j.1530-0277.1993.tb05668.x
Attias J, Raveh E, Aizer-Dannon A, Bloch-Mimouni A, Fattal-Valevski A (2012) Auditory system dysfunction due to infantile thiamine deficiency: long-term auditory sequelae. Audiol Neurotol 17:309–320. https://doi.org/10.1159/000339356
Pitkin SR, Savage LM (2004) Age-related vulnerability to diencephalic amnesia produced by thiamine deficiency: the role of time of insult. Behav Brain Res 148:93–105. https://doi.org/10.1016/S0166-4328(03)00208-0
Chaves RAD, do Couto TT, Valadares K, de O, Stringhini MLF (2007) Deficiências nutricionais pós-cirurgia bariátrica em adultos com obesidade mórbida. Rev Medica Minas Gerais 17:121–128
Torezan EFG (2013) Revisão das principais deficiências de micronutrientes no pós-operatório do Bypass Gástrico em Y de Roux. Int J Nutrol 6:37–42
Kumar N (2017) Nutrients and Neurology. Continuum (N Y) 23:822–861. https://doi.org/10.1212/01.CON.0000520630.69195.90
Sant’Ana FJF, Barros CSL (2010) Polioencephalomalacia in ruminants in Brazil. Braz J Vet Pathol 3:70–79
Miller AD, Zachary JF (2018) Sistema Nervoso. In: Zachary JF (ed) Bases da Patologia em Veterinária, 6th edn. Elsevier, Rio de Janeiro, pp 805–907
Nardone R, Höller Y, Storti M, Christova M, Tezzon F, Golaszewski S, Trinka E, Brigo F (2013) Thiamine deficiency induced neurochemical, neuroanatomical, and neuropsychological alterations: a reappraisal. Sci World J.https://doi.org/10.1155/2013/309143
Evans CA, Carlosn WE, Green RG (1942) The pathology of chastek paralysis in foxes: a counterpart of Wernicke’s hemorrhagic pollioencephalitis of man. Am J Pathol 18:79–91
Kumar N (2010) Neurologic presentations of nutritional deficiencies. Neurol Clin 28:107–170. https://doi.org/10.1016/j.ncl.2009.09.006
Victor M (1971) Deficiency diseases of the nervous system secondary to alcoholism. Postgrad Med 50:75–79. https://doi.org/10.1080/00325481.1971.11697591
Kril JJ (1996) Neuropathology of thiamine deficiency disorders. Metab Brain Dis 11:9–17
Cunha P, Badial P, Cagnini D, Oliveira-Filho J, Moares L, Takahira R, Amorim R, Borges A (2011) Polioencefalomalacia experimental em bovinos induzida por toxicose por enxofre. Pesqui Vet Bras 31:41–52
Liu D, Ke Z, Luo J (2017) Thiamine deficiency and neurodegeneration: the interplay among oxidative stress, endoplasmic reticulum stress, and autophagy. Mol Neurobiol 54:5440–5448. https://doi.org/10.1007/s12035-016-0079-9
Bâ A (2017) Alcohol and thiamine deficiency trigger differential mitochondrial transition pore opening mediating cellular death. Apoptosis 22:741–752. https://doi.org/10.1007/s10495-017-1372-4
Erikson KM, Thompson K, Aschner J, Aschner M (2007) Manganese neurotoxicity: a focus on the neonate. Pharmacol Ther 113:369–377. https://doi.org/10.1016/j.pharmthera.2006.09.002
Xu B, Xu Z-F, Deng Y (2010) Protective effects of MK-801 on manganese-induced glutamate metabolism disorder in rat striatum. Exp Toxicol Pathol 62:381–390. https://doi.org/10.1016/j.etp.2009.05.007
Roth J (2009) Are there common biochemical and molecular mechanisms controlling manganism and parkisonism. NeuroMol Med 11:281–296. https://doi.org/10.1007/s12017-009-8088-8
Chen J, Li C, Pei D-S, Han D, Liu X-M, Jiang H-X, Wang X-T, Guan Q-H, Wen X-R, Hou X-Y, Zhang G-Y (2009) GluR6-containing KA receptor mediates the activation of p38 MAP kinase in rat hippocampal CA1 region during brain ischemia injury. Hippocampus 19:79–89. https://doi.org/10.1002/hipo.20479
Rama Rao KV, Jayakumar AR, Reddy PVB, Tong X, Curtis KM, Norenberg MD (2010) Aquaporin-4 in manganese-treated cultured astrocytes. Glia 58:1490–1499. https://doi.org/10.1002/glia.21023
Aouadi M, Binetruy B, Caron L, Le Marchand-Brustel Y, Bost F (2006) Role of MAPKs in development and differentiation: lessons from knockout mice. Biochimie 88:1091–1098. https://doi.org/10.1016/j.biochi.2006.06.003
Thomas GM, Huganir RL (2004) MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci 5:173–183. https://doi.org/10.1038/nrn1346
Subramaniam S, Unsicker K (2006) Extracellular signal-regulated kinase as an inducer of non-apoptotic neuronal death. Neuroscience 138:1055–1065. https://doi.org/10.1016/j.neuroscience.2005.12.013
Cowan KJ, Storey KB (2003) Mitogen-activated protein kinases: new signaling pathways functioning in cellular responses to environmental stress. J Exp Biol 206:1107–1115. https://doi.org/10.1242/jeb.00220
Waetzig V, Herdegen T (2004) Neurodegenerative and physiological actions of c-Jun N-terminal kinases in the mammalian brain. Neurosci Lett 361:64–67. https://doi.org/10.1016/j.neulet.2004.02.041
Maamoun H, Benameur T, Pintus G, Munusamy S, Agouni A (2019) Crosstalk between oxidative stress and endoplasmic reticulum (ER) stress in endothelial dysfunction and aberrant angiogenesis associated with diabetes: a focus on the protective roles of heme oxygenase (HO)-1. Front Physiol.https://doi.org/10.3389/fphys.2019.00070
Keyse SM, Tyrrell RM (1989) Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci 86:99–103. https://doi.org/10.1073/pnas.86.1.99
Alam J, Igarashi K, Immenschuh S, Shibahara S, Tyrrell RM (2004) Regulation of heme oxygenase-1 gene transcription: recent advances and highlights from the International Conference (Uppsala, 2003) on Heme Oxygenase. Antioxid Redox Signal 6:924–33. https://doi.org/10.1089/ars.2004.6.924
Foresti R, Hoque M, Bains S, Green CJ, Motterlini R (2003) Haem and nitric oxide: synergism in the modulation of the endothelial haem oxygenase-1 pathway. Biochem J 372:381–390. https://doi.org/10.1042/BJ20021516
Barañano DE, Snyder SH (2001) Neural roles for heme oxygenase: contrasts to nitric oxide synthase. Proc Natl Acad Sci USA 98:10996–11002. https://doi.org/10.1073/pnas.191351298
Chen J (2014) Heme oxygenase in neuroprotection: from mechanisms to therapeutic implications. Rev Neurosci 25:269–280. https://doi.org/10.1515/revneuro-2013-0046
Calabrese V, Scapagnini G, Ravagna A, Fariello RG, Giuffrida Stella AM, Abraham NG (2002) Regional distribution of heme oxygenase, HSP70, and glutathione in brain: relevance for endogenous oxidant/antioxidant balance and stress tolerance. J Neurosci Res 68:65–75. https://doi.org/10.1002/jnr.10177
Scapagnini G, D’Agata V, Calabrese V, Pascale A, Colombrita C, Alkon D, Cavallaro S (2002) Gene expression profiles of heme oxygenase isoforms in the rat brain. Brain Res 954:51–59. https://doi.org/10.1016/s0006-8993(02)03338-3
Gozzelino R, Jeney V, Soares MP (2010) Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol 50:323–354. https://doi.org/10.1146/annurev.pharmtox.010909.105600
Loboda A, Jozkowicz A, Dulak J (2015) HO-1/CO system in tumor growth, angiogenesis and metabolism—targeting HO-1 as an anti-tumor therapy. Vascul Pharmacol 74:11–22. https://doi.org/10.1016/j.vph.2015.09.004
Nitti M, Piras S, Brondolo L, Marinari U, Pronzato M, Furfaro A (2018) Heme oxygenase 1 in the nervous system: does it favor neuronal cell survival or induce neurodegeneration? Int J Mol Sci 19:2260. https://doi.org/10.3390/ijms19082260
Calingasan NY, Gibson GE (2000) Dietary restriction attenuates the neuronal loss, induction of heme oxygenase-1 and blood–brain barrier breakdown induced by impaired oxidative metabolism. Brain Res 885:62–69. https://doi.org/10.1016/S0006-8993(00)02933-4
Calingasan NY, Chun WJ, Park LCH, Uchida K, Gibson GE (1999) Oxidative stress is associated with region-specific neuronal death during thiamine deficiency. J Neuropathol Exp Neurol 58:946–958. https://doi.org/10.1097/00005072-199909000-00005
Ke Z-J, Degiorgio LA, Volpe BT, Gibson GE (2003) Reversal of thiamine deficiency-induced neurodegeneration. J Neuropathol Exp Neurol 62:195–207. https://doi.org/10.1093/jnen/62.2.195
Wu T-W, Hashimoto N, Au J-X, Wu J, Mickle DAG, Carey D (1991) Trolox protects rat hepatocytes against oxyradical damage and the ischemic rat liver from reperfusion injury. Hepatology 13:575–580. https://doi.org/10.1002/hep.1840130328
Albertini R, Abuja PM (1999) Prooxidant and antioxidant properties of Trolox C, analogue of vitamin E, in oxidation of low-density lipoprotein. Free Radic Res 30:181–188. https://doi.org/10.1080/10715769900300201
Wu T-W, Hashimoto N, Wu J, Carey D, Li R-K, Mickle DAG, Weisel RD (1990) The cytoprotective effect of Trolox demonstrated with three types of human cells. Biochem Cell Biol 68:1189–1194. https://doi.org/10.1139/o90-176
Sagach VF, Scrosati M, Fielding J, Rossoni G, Galli C, Visioli F (2002) The water-soluble vitamin E analogue Trolox protects against ischaemia/reperfusion damage in vitro and ex vivo. A comparison with vitamin E. Pharmacol Res 45:435–439. https://doi.org/10.1006/phrs.2002.0993
Jacob SW, de la Torre JC (2009) Pharmacology of dimethyl sulfoxide in cardiac and CNS damage. Pharmacol Rep 61:225–235. https://doi.org/10.1016/S1734-1140(09)70026-X
Santos NC, Figueira-Coelho J, Martins-Silva J, Saldanha C (2003) Multidisciplinary utilization of dimethyl sulfoxide: pharmacological, cellular, and molecular aspects. Biochem Pharmacol 65:1035–1041. https://doi.org/10.1016/S0006-2952(03)00002-9
Brayton CF (1986) Dimethyl sulfoxide (DMSO): a review. Cornell Vet 76:61–90
Blythe LL, Craig AM, Christensen JM, Appell LH, Slizeski ML (1986) Pharmacokinetic disposition of dimethyl sulfoxide administered intravenously to horses. Am J Vet Res 47:1739–1743
Cordova FM, Aguiar AS, Peres TV, Lopes MW, Goncalves FM, Remor AP, Lopes SC, Pilati C, Latini AS, Prediger RD, Erikson KM, Aschner M, Leal RB (2012) In vivo manganese exposure modulates Erk, Akt and Darpp-32 in the striatum of developing rats, and impairs their motor function. PLoS ONE 7:e33057. https://doi.org/10.1371/journal.pone.0033057
Cordova FM, Aguiar AS, Peres TV, Lopes MW, Gonçalves FM, Pedro DZ, Lopes SC, Pilati C, Prediger RDS, Farina M, Erikson KM, Aschner M, Leal RB (2013) Manganese-exposed developing rats display motor deficits and striatal oxidative stress that are reversed by Trolox. Arch Toxicol 87:1231–1244. https://doi.org/10.1007/s00204-013-1017-5
Hazell AS, Butterworth RF (2009) Update of cell damage mechanisms in thiamine deficiency: focus on oxidative stress, excitotoxicity and inflammation. Alcohol Alcohol 44:141–147. https://doi.org/10.1093/alcalc/agn120
Zhang SX, Weilersbacher GS, Henderson SW, Corso T, Olney JW, Langlais PJ (1995) Excitotoxic cytopathology, progression, and reversibility of thiamine deficiency-induced diencephalic lesions. J Neuropathol Exp Neurol 54:255–267. https://doi.org/10.1097/00005072-199503000-00012
Langlais PJ (1995) Pathogenesis of diencephalic lesions in an experimental model of Wernicke’s encephalopathy. Metab Brain Dis 10:31–44. https://doi.org/10.1007/BF01991781
Reeves PG, Nielsen FH, Fahey GC (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J Nutr 123:1939–1951. https://doi.org/10.1093/jn/123.11.1939
Calingasan N, Gandy S, Baker H, Sheu KF, Smith JD, Lamb BT, Gearhart JD, Buxbaum JD, Harper C, Selkoe DJ, Price DL, Sisodia SS, Gibson GE (1996) Novel neuritic clusters with accumulations of amyloid precursor protein and amyloid precursor-like protein 2 immunoreactivity in brain regions damaged by thiamine. Am J Pathol 149:1063–1071
Cordova FM, Rodrigues ALS, Giacomelli MBO, Oliveira CS, Posser T, Dunkley PR, Leal RB (2004) Lead stimulates ERK1/2 and p38MAPK phosphorylation in the hippocampus of immature rats. Brain Res 998:65–72. https://doi.org/10.1016/j.brainres.2003.11.012
Cavas M, Beltrán D, Navarro JF (2005) Behavioural effects of dimethyl sulfoxide (DMSO): changes in sleep architecture in rats. Toxicol Lett 157:221–232. https://doi.org/10.1016/j.toxlet.2005.02.003
Pereira LM, Aguiar HQ, da Rodrigues S, Moraes SDC, Medeiros JO, de CN R, de Cordova CAS, de Cordova FM (2017) Amprolium-induced thiamine deficiency in mice: evaluation of a practical model by oral administration. Acta Vet Bras 11:164–174. https://doi.org/10.21708/avb.2017.11.0.7101
Moraes JO, Rodrigues SDC, Pereira LM, Medeiros R, dede CNCordova CAS, de Cordova FM (2018) Amprolium exposure alters mice behavior and metabolism in vivo. Anim Model Exp Med 1:272–281. https://doi.org/10.1002/ame2.12040
Aguiar AS, Araújo AL, Da-Cunha TR, Speck AE, Ignácio ZM, De-Mello N, Prediger RDS (2009) Physical exercise improves motor and short-term social memory deficits in reserpinized rats. Brain Res Bull 79:452–457. https://doi.org/10.1016/j.brainresbull.2009.05.005
Peterson GL (1977) A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 83:346–356. https://doi.org/10.1016/0003-2697(77)90043-4
Leal RB, Cordova FM, Herd L, Bobrovskaya L, Dunkley PR (2002) Lead-stimulated p38MAPK-dependent Hsp27 phosphorylation. Toxicol Appl Pharmacol 178:44–51. https://doi.org/10.1006/taap.2001.9320
Cordova CAS, Locatelli C, Assunção LS, Mattei B, Mascarello A, Winter E, Nunes RJ, Yunes RA, Creczynski-Pasa TB (2011) Octyl and dodecyl gallates induce oxidative stress and apoptosis in a melanoma cell line. Toxicol Vitr 25:2025–2034. https://doi.org/10.1016/j.tiv.2011.08.003
Posser T, de Aguiar CNM, Garcez R, Rossi F, Oliveira C, Trentin A, Moura Neto V, Leal R (2007) Exposure of C6 glioma cells to Pb(II) increases the phosphorylation of p38MAPK and JNK1/2 but not of ERK1/2. Arch Toxicol 81:407–414. https://doi.org/10.1007/s00204-007-0177-6
Sheline CT, Wei L (2006) Free radical-mediated neurotoxicity may be caused by inhibition of mitochondrial dehydrogenases in vitro and in vivo. Neuroscience 140:235–246. https://doi.org/10.1016/j.neuroscience.2006.02.019
Gao X, Zhang H, Takahashi T, Hsieh J, Liao J, Steinberg GK, Zhao H (2008) The Akt signaling pathway contributes to postconditioning’s protection against stroke; the protection is associated with the MAPK and PKC pathways. J Neurochem 105:943–955. https://doi.org/10.1111/j.1471-4159.2008.05218.x
Tolosa EMC de, Rodrigues CJ, Behmer OA, Neto AG de F (2003) Manual de Técnicas para Histologia Normal e Patológica, 2nd edn. Manole, Barueri
Franklin KBJ, Paxinos G (1997) The mouse brain in stereotaxic coordinates. Academic Press, San Diego
Schwaiger J, Wanke R, Adam S, Pawert M, Honnen W, Triebskorn R (1997) The use of histopathological indicators to evaluate contaminant-related stress in fish. J Aquat Ecosyst Stress Recover 6:75–86. https://doi.org/10.1023/A:1008212000208
Anjaneya A, Singh KP, Cherian S, Saminathan M, Singh R, Ramakrishnan MA, Maan S, Maan NS, Hemadri D, Rao PP, Putty K, Krishnajyothi Y, Mertens PP (2018) Comparative neuropathology of major indian bluetongue virus serotypes in a neonatal BALB/c mouse model. J Comp Pathol 162:18–28. https://doi.org/10.1016/j.jcpa.2018.06.001
Holland T, Holland C (2011) Analysis of unbiased histopathology data from rodent toxicity studies (or, are these groups different enough to ascribe it to treatment?). Toxicol Pathol 39:569–575. https://doi.org/10.1177/0192623311406289
Crissman JW, Goodman DG, Hildebrandt PK, Maronpot RR, Prater DA, Riley JH, Seaman WJ, Thake DC (2004) Best practices guideline: toxicologic histopathology. Toxicol Pathol 32:126–131. https://doi.org/10.1080/01926230490268756
Manzetti S, Zhang J, Van Der Spoel D (2014) Thiamin function, metabolism, uptake, and transport. Biochemistry 53:821–835. https://doi.org/10.1021/bi401618y
Chornyy S, Parkhomenko J, Chorna N (2007) Thiamine deficiency caused by thiamine antagonists triggers upregulation of apoptosis inducing factor gene expression and leads to caspase 3-mediated apoptosis in neuronally differentiated rat PC-12 cells. Acta Biochim Pol 54:315–322
Wang X, Wang B, Fan Z, Shi X, Ke Z-J, Luo J (2007) Thiamine deficiency induces endoplasmic reticulum stress in neurons. Neuroscience 144:1045–1056. https://doi.org/10.1016/j.neuroscience.2006.10.008
Liu M, Alimov AP, Wang H, Frank JA, Katz W, Xu M, Ke Z-J, Luo J (2014) Thiamine deficiency induces anorexia by inhibiting hypothalamic AMPK. Neuroscience 267:102–113. https://doi.org/10.1016/j.neuroscience.2014.02.033
Bâ A (2012) Effects of thiamine deficiency on food intake and body weight increment in adult female and growing rats. Behav Pharmacol 23:575–581. https://doi.org/10.1097/FBP.0b013e32835724a1
Li W, Ji M, Lin Y, Miao Y, Chen S, Li H (2018) DEPP/DEPP1/C10ORF10 regulates hepatic glucose and fat metabolism partly via ROS-induced FGF21. FASEB J.https://doi.org/10.1096/fj.201800357R
Kabel AM, Alzahrani AA, Bawazir NM, Khawtani RO, Arab HH (2018) Targeting the proinflammatory cytokines, oxidative stress, apoptosis and TGF-β1/STAT-3 signaling by irbesartan to ameliorate doxorubicin-induced hepatotoxicity. J Infect Chemother.https://doi.org/10.1016/j.jiac.2018.03.010
Dangarembizi R, Erlwanger KH, Rummel C, Roth J, Madziva MT, Harden LM (2018) Brewer’s yeast is a potent inducer of fever, sickness behavior and inflammation within the brain. Brain Behav Immun 68:211–223. https://doi.org/10.1016/j.bbi.2017.10.019
Le Thuc O, Stobbe K, Cansell C, Nahon J-L, Blondeau N, Rovère C (2017) Hypothalamic inflammation and energy balance disruptions: spotlight on chemokines. Front Endocrinol (Lausanne) 8:197. https://doi.org/10.3389/fendo.2017.00197
Ferreira-Vieira TH, Freitas-Silva DM de, Ribeiro AF, Pereira SRC, Ribeiro ÂM (2016) Perinatal thiamine restriction affects central GABA and glutamate concentrations and motor behavior of adult rat offspring. Neurosci Lett 617:182–187. https://doi.org/10.1016/j.neulet.2016.01.060
Carvalho FM, Pereira SRC, Pires RGW, Ferraz VP, Romano-Silva MA, Oliveira-Silva IF, Ribeiro AM (2006) Thiamine deficiency decreases glutamate uptake in the prefrontal cortex and impairs spatial memory performance in a water maze test. Pharmacol Biochem Behav 83:481–489. https://doi.org/10.1016/j.pbb.2006.03.004
Bowyer JF, Tranter KM, Sarkar S, Hanig JP (2018) Microglial activation and vascular responses that are associated with early thalamic neurodegeneration resulting from thiamine deficiency. Neurotoxicology 65:98–110. https://doi.org/10.1016/j.neuro.2018.02.005
Colucci M, Maione F, Bonito MC, Piscopo A, Di Giannuario A, Pieretti S (2008) New insights of dimethyl sulphoxide effects (DMSO) on experimental in vivo models of nociception and inflammation. Pharmacol Res 57:419–425. https://doi.org/10.1016/j.phrs.2008.04.004
Ichinohe N, Mori F, Shoumura K (2000) A di-synaptic projection from the lateral cerebellar nucleus to the laterodorsal part of the striatum via the central lateral nucleus of the thalamus in the rat. Brain Res 880:191–197. https://doi.org/10.1016/S0006-8993(00)02744-X
Uusisaari M, Obata K, Knöpfel T (2007) Morphological and electrophysiological properties of GABAergic and non-GABAergic cells in the deep cerebellar nuclei. J Neurophysiol 97:901–911. https://doi.org/10.1152/jn.00974.2006
Langlais PJ, Anderson G, Guo SX, Bondy SC (1997) Increased cerebral free radical production during thiamine deficiency. Metab Brain Dis 12:137–143. https://doi.org/10.1007/BF02674735
Desjardins P, Butterworth RF (2005) Role of mitochondrial dysfunction and oxidative stress in the pathogenesis of selective neuronal loss in Wernicke’s encephalopathy. Mol Neurobiol 31:17–25. https://doi.org/10.1385/MN:31:1-3:017
Zuccoli G, Pipitone N (2009) Neuroimaging findings in acute Wernicke’s encephalopathy: review of the literature. Am J Roentgenol 192:501–508. https://doi.org/10.2214/AJR.07.3959
Todd KG, Butterworth RF (1998) Evaluation of the role of NMDA-mediated excitotoxicity in the selective neuronal loss in experimental Wernicke encephalopathy. Exp Neurol 149:130–138. https://doi.org/10.1006/exnr.1997.6677
Todd KG, Butterworth RF (1999) Early microglial response in experimental thiamine deficiency: an immunohistochemical analysis. Glia 25:190–198
Langlais P, Mair R (1990) Protective effects of the glutamate antagonist MK-801 on pyrithiamine- induced lesions and amino acid changes in rat brain. J Neurosci 10:1664–1674. https://doi.org/10.1523/JNEUROSCI.10-05-01664.1990
Rigon AP, Cordova FM, Oliveira CS, Posser T, Costa AP, Silva IG, Santos DA, Rossi FM, Rocha JBT, Leal RB (2008) Neurotoxicity of cadmium on immature hippocampus and a neuroprotective role for p38MAPK. Neurotoxicology 29:727–734. https://doi.org/10.1016/j.neuro.2008.04.017
Molz S, Decker H, Dal-Cim T, Cremonez C, Cordova FM, Leal RB, Tasca CI (2008) Glutamate-induced toxicity in hippocampal slices involves apoptotic features and p38 MAPK signaling. Neurochem Res 33:27–36. https://doi.org/10.1007/s11064-007-9402-1
Wang JJ-L, Hua Z, Fentress HM, Singleton CK (2000) JNK1 is inactivated during thiamine deficiency-induced apoptosis in human neuroblastoma cells. J Nutr Biochem 11:208–215. https://doi.org/10.1016/S0955-2863(00)00067-X
Kawakami Z, Ikarashi Y, Kase Y (2010) Glycyrrhizin and its metabolite 18 beta-glycyrrhetinic acid in glycyrrhiza, a constituent herb of yokukansan, ameliorate thiamine deficiency-induced dysfunction of glutamate transport in cultured rat cortical astrocytes. Eur J Pharmacol 626:154–158. https://doi.org/10.1016/j.ejphar.2009.09.046
Schipper H, Song W (2015) A heme oxygenase-1 transducer model of degenerative and developmental brain disorders. Int J Mol Sci 16:5400–5419. https://doi.org/10.3390/ijms16035400
Schipper HM, Song W, Tavitian A, Cressatti M (2019) The sinister face of heme oxygenase-1 in brain aging and disease. Prog Neurobiol 172:40–70. https://doi.org/10.1016/j.pneurobio.2018.06.008
Schipper HM, Song W, Zukor H, Hascalovici JR, Zeligman D (2009) Heme oxygenase-1 and neurodegeneration: expanding frontiers of engagement. J Neurochem 110:469–485. https://doi.org/10.1111/j.1471-4159.2009.06160.x
Schipper HM (2004) Heme oxygenase expression in human central nervous system disorders. Free Radic Biol Med 37:1995–2011. https://doi.org/10.1016/j.freeradbiomed.2004.09.015
Aggeli I-K, Theofilatos D, Beis I, Gaitanaki C (2011) Insulin-induced oxidative stress up-regulates heme oxygenase-1 via diverse signaling cascades in the C2 skeletal myoblast cell line. Endocrinology 152:1274–1283. https://doi.org/10.1210/en.2010-1319
Canas N, Valero T, Villarroya M, Montell E, Verges J, Garcia AG, Lopez MG (2007) Chondroitin sulfate protects SH-SY5Y cells from oxidative stress by inducing heme oxygenase-1 via phosphatidylinositol 3-kinase/Akt. J Pharmacol Exp Ther 323:946–953. https://doi.org/10.1124/jpet.107.123505
Engel DF, de Oliveira J, Lieberknecht V, Rodrigues ALS, de Bem AF, Gabilan NH (2018) Duloxetine protects human neuroblastoma cells from oxidative stress-induced cell death through Akt/Nrf-2/HO-1 pathway. Neurochem Res 43:387–396. https://doi.org/10.1007/s11064-017-2433-3
Aggeli I-KS, Gaitanaki C, Beis I (2006) Involvement of JNKs and p38-MAPK/MSK1 pathways in H2O2-induced upregulation of heme oxygenase-1 mRNA in H9c2 cells. Cell Signal 18:1801–1812. https://doi.org/10.1016/j.cellsig.2006.02.001
Zhang X, Bedard EL, Potter R, Zhong R, Alam J, Choi AMK, Lee PJ (2002) Mitogen-activated protein kinases regulate HO-1 gene transcription after ischemia-reperfusion lung injury. Am J Physiol Lung Cell Mol Physiol 283:L815–L829. https://doi.org/10.1152/ajplung.00485.2001
Wijayanti N, Kietzmann T, Immenschuh S (2005) Heme oxygenase-1 gene activation by the NAD(P)H oxidase inhibitor 4-(2-aminoethyl) benzenesulfonyl fluoride via a protein kinase B, p38-dependent signaling pathway in monocytes. J Biol Chem 280:21820–21829. https://doi.org/10.1074/jbc.M502943200
Doré S, Sampei K, Goto S, Alkayed NJ, Guastella D, Blackshaw S, Gallagher M, Traystman RJ, Hurn PD, Koehler RC, Snyder SH (1999) Heme oxygenase-2 is neuroprotective in cerebral ischemia. Mol Med 5:656–663
Acknowledgements
This study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil (Grant Number 470252/2013-8). L. M. Pereira, S. D. C. Rodrigues, and H. Q. S. Aguiar are fellows from the Institutional Program of Scientific Initiation Scholarships of the Universidade Federal do Tocantins (PIBIC/CNPq/UFT). We would like to thank Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Contributions
All listed authors meet the requirements for authorship. RCNM, AYJr, CASC and FMC conceived and designed the experiments; RCNM, JOM, LMP, SDCR, HQSA and FMC performed the experiments. RCNM, AYJr, CASC and FMC performed the experiments and wrote the main manuscript text. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any financial or personal relationships that could inappropriately influence or bias the content of the paper.
Ethical Approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted (Universidade Federal do Tocantins Ethics Committee on Animal Use, CEUA-UFT, Permit Number 23101.000284/2014-13).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Medeiros, R.C.N., Moraes, J.O., Rodrigues, S.D.C. et al. Thiamine Deficiency Modulates p38MAPK and Heme Oxygenase-1 in Mouse Brain: Association with Early Tissue and Behavioral Changes. Neurochem Res 45, 940–955 (2020). https://doi.org/10.1007/s11064-020-02975-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-02975-7