Abstract
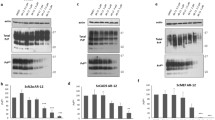
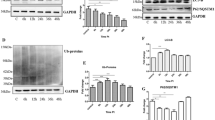
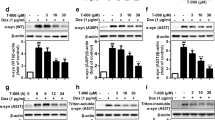
Prion diseases are fatal infectious neurodegenerative disorders in human and animals caused by misfolding of the cellular prion protein (PrPC) into the infectious isoform PrPSc. These diseases have the potential to transmit within or between species, and no cure is available to date. Targeting the unfolded protein response (UPR) as an anti-prion therapeutic approach has been widely reported for prion diseases. Here, we describe the anti-prion effect of the chemical compound Sephin1 which has been shown to protect in mouse models of protein misfolding diseases including amyotrophic lateral sclerosis (ALS) and multiple sclerosis (MS) by selectively inhibiting the stress-induced regulatory subunit of protein phosphatase 1, thus prolonging eIF2α phosphorylation. We show here that Sephin1 dose and time dependently reduced PrPSc in different neuronal cell lines which were persistently infected with various prion strains. In addition, prion seeding activity was reduced in Sephin1-treated cells. Importantly, we found that Sephin1 significantly overcame the endoplasmic reticulum (ER) stress induced in treated cells, as measured by lower expression of stress-induced aberrant prion protein. In a mouse model of prion infection, intraperitoneal treatment with Sephin1 significantly prolonged survival of prion-infected mice. When combining Sephin1 with the neuroprotective drug metformin, the survival of prion-infected mice was also prolonged. These results suggest that Sephin1 could be a potential anti-prion drug selectively targeting one component of the UPR pathway.






Similar content being viewed by others
References
Prusiner SB (1982) Novel proteinaceous infectious particles cause scrapie. Science 216(4542):136–144. https://doi.org/10.1126/science.6801762
Prusiner SB (1998) Prions. Proc Natl Acad Sci U S A 95(23):13363–13383
Prusiner SB (2013) Biology and genetics of prions causing neurodegeneration. Annu Rev Genet 47:601–623. https://doi.org/10.1146/annurev-genet-110711-155524
DeMarco ML, Daggett V (2004) From conversion to aggregation: protofibril formation of the prion protein. Proc Natl Acad Sci U S A 101(8):2293–2298. https://doi.org/10.1073/pnas.0307178101
Budka H (2003) Neuropathology of prion diseases. Br Med Bull 66:121–130. https://doi.org/10.1093/bmb/66.1.121
Pan KM, Baldwin M, Nguyen J, Gasset M, Serban A, Groth D, Mehlhorn I, Huang Z et al (1993) Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci U S A 90(23):10962–10966. https://doi.org/10.1073/pnas.90.23.10962
Smirnovas V, Baron GS, Offerdahl DK, Raymond GJ, Caughey B, Surewicz WK (2011) Structural organization of brain-derived mammalian prions examined by hydrogen-deuterium exchange. Nat Struct Mol Biol 18(4):504–506. https://doi.org/10.1038/nsmb.2035
Wille H, Bian W, McDonald M, Kendall A, Colby DW, Bloch L, Ollesch J, Borovinskiy AL et al (2009) Natural and synthetic prion structure from X-ray fiber diffraction. Proc Natl Acad Sci U S A 106(40):16990–16995. https://doi.org/10.1073/pnas.0909006106
Madec JY, Vanier A, Dorier A, Bernillon J, Belli P, Baron T (1997) Biochemical properties of protease resistant prion protein PrPsc in natural sheep scrapie. Arch Virol 142(8):1603–1612
Collinge J (2005) Molecular neurology of prion disease. J Neurol Neurosurg Psychiatry 76(7):906–919. https://doi.org/10.1136/jnnp.2004.048660
Collinge J (2001) Prion diseases of humans and animals: their causes and molecular basis. Annu Rev Neurosci 24:519–550. https://doi.org/10.1146/annurev.neuro.24.1.519
Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A, McCardle L, Chree A et al (1997) Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature 389(6650):498–501. https://doi.org/10.1038/39057
Hill AF, Desbruslais M, Joiner S, Sidle KC, Gowland I, Collinge J, Doey LJ, Lantos P (1997) The same prion strain causes vCJD and BSE. Nature 389(6650):448–450, 526. https://doi.org/10.1038/38925
Tatzelt J, Winklhofer KF (2004) Folding and misfolding of the prion protein in the secretory pathway. Amyloid : the international journal of experimental and clinical investigation : the official journal of the International Society of Amyloidosis 11(3):162–172
Soto C, Satani N (2011) The intricate mechanisms of neurodegeneration in prion diseases. Trends Mol Med 17(1):14–24. https://doi.org/10.1016/j.molmed.2010.09.001
Hetz CA, Soto C (2006) Stressing out the ER: a role of the unfolded protein response in prion-related disorders. Curr Mol Med 6(1):37–43
Heiseke A, Aguib Y, Schatzl HM (2010) Autophagy, prion infection and their mutual interactions. Current issues in molecular biology 12(2):87–97
Torres M, Castillo K, Armisen R, Stutzin A, Soto C, Hetz C (2010) Prion protein misfolding affects calcium homeostasis and sensitizes cells to endoplasmic reticulum stress. PLoS One 5(12):e15658. https://doi.org/10.1371/journal.pone.0015658
Orsi A, Fioriti L, Chiesa R, Sitia R (2006) Conditions of endoplasmic reticulum stress favor the accumulation of cytosolic prion protein. J Biol Chem 281(41):30431–30438. https://doi.org/10.1074/jbc.M605320200
Hetz C, Castilla J, Soto C (2007) Perturbation of endoplasmic reticulum homeostasis facilitates prion replication. J Biol Chem 282(17):12725–12733. https://doi.org/10.1074/jbc.M611909200
Rane NS, Kang SW, Chakrabarti O, Feigenbaum L, Hegde RS (2008) Reduced translocation of nascent prion protein during ER stress contributes to neurodegeneration. Dev Cell 15(3):359–370. https://doi.org/10.1016/j.devcel.2008.06.015
Nunziante M, Ackermann K, Dietrich K, Wolf H, Gadtke L, Gilch S, Vorberg I, Groschup M et al (2011) Proteasomal dysfunction and endoplasmic reticulum stress enhance trafficking of prion protein aggregates through the secretory pathway and increase accumulation of pathologic prion protein. J Biol Chem 286(39):33942–33953. https://doi.org/10.1074/jbc.M111.272617
Moreno JA, Radford H, Peretti D, Steinert JR, Verity N, Martin MG, Halliday M, Morgan J et al (2012) Sustained translational repression by eIF2alpha-P mediates prion neurodegeneration. Nature 485(7399):507–511. https://doi.org/10.1038/nature11058
Broadley SA, Hartl FU (2009) The role of molecular chaperones in human misfolding diseases. FEBS Lett 583(16):2647–2653. https://doi.org/10.1016/j.febslet.2009.04.029
Park KW, Eun Kim G, Morales R, Moda F, Moreno-Gonzalez I, Concha-Marambio L, Lee AS, Hetz C et al (2017) The endoplasmic reticulum chaperone GRP78/BiP modulates prion propagation in vitro and in vivo. Sci Rep 7:44723. https://doi.org/10.1038/srep44723
Thapa S, Abdulrahman B, Abdelaziz DH, Lu L, Ben Aissa M, Schatzl HM (2018) Overexpression of quality control proteins reduces prion conversion in prion-infected cells. J Biol Chem 293(41):16069–16082. https://doi.org/10.1074/jbc.RA118.002754
Crunkhorn S (2015) Neurodegenerative disease: phosphatase inhibitor prevents protein-misfolding diseases. Nat Rev Drug Discov 14(6):386. https://doi.org/10.1038/nrd4638
Das I, Krzyzosiak A, Schneider K, Wrabetz L, D’Antonio M, Barry N, Sigurdardottir A, Bertolotti A (2015) Preventing proteostasis diseases by selective inhibition of a phosphatase regulatory subunit. Science 348(6231):239–242. https://doi.org/10.1126/science.aaa4484
Chen Y, Podojil JR, Kunjamma RB, Jones J, Weiner M, Lin W, Miller SD, Popko B (2019) Sephin1, which prolongs the integrated stress response, is a promising therapeutic for multiple sclerosis. Brain : a journal of neurology 142(2):344–361. https://doi.org/10.1093/brain/awy322
Jiang HQ, Ren M, Jiang HZ, Wang J, Zhang J, Yin X, Wang SY, Qi Y et al (2014) Guanabenz delays the onset of disease symptoms, extends lifespan, improves motor performance and attenuates motor neuron loss in the SOD1 G93A mouse model of amyotrophic lateral sclerosis. Neuroscience 277:132–138. https://doi.org/10.1016/j.neuroscience.2014.03.047
Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D et al (2005) A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 307(5711):935–939. https://doi.org/10.1126/science.1101902
Tsaytler P, Harding HP, Ron D, Bertolotti A (2011) Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science 332(6025):91–94. https://doi.org/10.1126/science.1201396
Way SW, Podojil JR, Clayton BL, Zaremba A, Collins TL, Kunjamma RB, Robinson AP, Brugarolas P et al (2015) Pharmaceutical integrated stress response enhancement protects oligodendrocytes and provides a potential multiple sclerosis therapeutic. Nat Commun 6:6532. https://doi.org/10.1038/ncomms7532
Sun X, Aime P, Dai D, Ramalingam N, Crary JF, Burke RE, Greene LA, Levy OA (2018) Guanabenz promotes neuronal survival via enhancement of ATF4 and parkin expression in models of Parkinson disease. Exp Neurol 303:95–107. https://doi.org/10.1016/j.expneurol.2018.01.015
Tribouillard-Tanvier D, Beringue V, Desban N, Gug F, Bach S, Voisset C, Galons H, Laude H et al (2008) Antihypertensive drug guanabenz is active in vivo against both yeast and mammalian prions. PLoS One 3(4):e1981. https://doi.org/10.1371/journal.pone.0001981
Holmes B, Brogden RN, Heel RC, Speight TM, Avery GS (1983) Guanabenz. A review of its pharmacodynamic properties and therapeutic efficacy in hypertension. Drugs 26(3):212–229. https://doi.org/10.2165/00003495-198326030-00003
Cheng YC, Hannaoui S, John TR, Dudas S, Czub S, Gilch S (2016) Early and non-invasive detection of chronic wasting disease prions in elk feces by real-time quaking induced conversion. PLoS One 11(11):e0166187. https://doi.org/10.1371/journal.pone.0166187
Decker T, Lohmann-Matthes ML (1988) A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J Immunol Methods 115(1):61–69
Wang H, Wang X, Ke ZJ, Comer AL, Xu M, Frank JA, Zhang Z, Shi X et al (2015) Tunicamycin-induced unfolded protein response in the developing mouse brain. Toxicol Appl Pharmacol 283(3):157–167. https://doi.org/10.1016/j.taap.2014.12.019
Rotermund C, Machetanz G, Fitzgerald JC (2018) The therapeutic potential of metformin in neurodegenerative diseases. Front Endocrinol 9:400. https://doi.org/10.3389/fendo.2018.00400
Hetz C, Mollereau B (2014) Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat Rev Neurosci 15(4):233–249. https://doi.org/10.1038/nrn3689
Hoozemans JJ, van Haastert ES, Nijholt DA, Rozemuller AJ, Eikelenboom P, Scheper W (2009) The unfolded protein response is activated in pretangle neurons in Alzheimer’s disease hippocampus. Am J Pathol 174(4):1241–1251. https://doi.org/10.2353/ajpath.2009.080814
Jiang P, Gan M, Ebrahim AS, Lin WL, Melrose HL, Yen SH (2010) ER stress response plays an important role in aggregation of alpha-synuclein. Mol Neurodegener 5:56. https://doi.org/10.1186/1750-1326-5-56
Carnemolla A, Fossale E, Agostoni E, Michelazzi S, Calligaris R, De Maso L, Del Sal G, MacDonald ME et al (2009) Rrs1 is involved in endoplasmic reticulum stress response in Huntington disease. J Biol Chem 284(27):18167–18173. https://doi.org/10.1074/jbc.M109.018325
Atkin JD, Farg MA, Turner BJ, Tomas D, Lysaght JA, Nunan J, Rembach A, Nagley P et al (2006) Induction of the unfolded protein response in familial amyotrophic lateral sclerosis and association of protein-disulfide isomerase with superoxide dismutase 1. J Biol Chem 281(40):30152–30165. https://doi.org/10.1074/jbc.M603393200
Kaufman RJ (1999) Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev 13(10):1211–1233. https://doi.org/10.1101/gad.13.10.1211
Tabas I, Ron D (2011) Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 13(3):184–190. https://doi.org/10.1038/ncb0311-184
Gardner BM, Walter P (2011) Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science 333(6051):1891–1894. https://doi.org/10.1126/science.1209126
Cabral-Miranda F, Hetz C (2018) ER stress and neurodegenerative disease: a cause or effect relationship. Curr Top Microbiol Immunol 414:131–157. https://doi.org/10.1007/82_2017_52
Keene CD, Rodrigues CM, Eich T, Chhabra MS, Steer CJ, Low WC (2002) Tauroursodeoxycholic acid, a bile acid, is neuroprotective in a transgenic animal model of Huntington’s disease. Proc Natl Acad Sci U S A 99(16):10671–10676. https://doi.org/10.1073/pnas.162362299
Lindquist SL, Kelly JW (2011) Chemical and biological approaches for adapting proteostasis to ameliorate protein misfolding and aggregation diseases: progress and prognosis. Cold Spring Harb Perspect Biol 3(12). https://doi.org/10.1101/cshperspect.a004507
Reijonen S, Putkonen N, Norremolle A, Lindholm D, Korhonen L (2008) Inhibition of endoplasmic reticulum stress counteracts neuronal cell death and protein aggregation caused by N-terminal mutant huntingtin proteins. Exp Cell Res 314(5):950–960. https://doi.org/10.1016/j.yexcr.2007.12.025
Devi L, Ohno M (2014) PERK mediates eIF2alpha phosphorylation responsible for BACE1 elevation, CREB dysfunction and neurodegeneration in a mouse model of Alzheimer’s disease. Neurobiol Aging 35(10):2272–2281. https://doi.org/10.1016/j.neurobiolaging.2014.04.031
Teng Y, Gao M, Wang J, Kong Q, Hua H, Luo T, Jiang Y (2014) Inhibition of eIF2alpha dephosphorylation enhances TRAIL-induced apoptosis in hepatoma cells. Cell Death Dis 5:e1060. https://doi.org/10.1038/cddis.2014.24
Schmitz M, Cramm M, Llorens F, Candelise N, Muller-Cramm D, Varges D, Schulz-Schaeffer WJ, Zafar S et al (2016) Application of an in vitro-amplification assay as a novel pre-screening test for compounds inhibiting the aggregation of prion protein scrapie. Sci Rep 6:28711. https://doi.org/10.1038/srep28711
Abdelaziz DH, Thapa S, Abdulrahman B, Lu L, Jain S, Schatzl HM (2017) Immunization of cervidized transgenic mice with multimeric deer prion protein induces self-antibodies that antagonize chronic wasting disease infectivity in vitro. Sci Rep 7(1):10538. https://doi.org/10.1038/s41598-017-11235-8
Turner PV, Brabb T, Pekow C, Vasbinder MA (2011) Administration of substances to laboratory animals: routes of administration and factors to consider. Journal of the American Association for Laboratory Animal Science : JAALAS 50(5):600–613
Halliday M, Radford H, Zents KAM, Molloy C, Moreno JA, Verity NC, Smith E, Ortori CA et al (2017) Repurposed drugs targeting eIF2α-P-mediated translational repression prevent neurodegeneration in mice. Brain : a journal of neurology 140(6):1768–1783. https://doi.org/10.1093/brain/awx074
Moreno JA, Halliday M, Molloy C, Radford H, Verity N, Axten JM, Ortori CA, Willis AE, Fischer PM, Barrett DA, Mallucci GR (2013) Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Science translational medicine 5 (206):206ra138 doi:https://doi.org/10.1126/scitranslmed.3006767
Halliday M, Radford H, Sekine Y, Moreno J, Verity N, le Quesne J, Ortori CA, Barrett DA et al (2015) Partial restoration of protein synthesis rates by the small molecule ISRIB prevents neurodegeneration without pancreatic toxicity. Cell Death Dis 6:e1672. https://doi.org/10.1038/cddis.2015.49
Markowicz-Piasecka M, Sikora J, Szydlowska A, Skupien A, Mikiciuk-Olasik E, Huttunen KM (2017) Metformin - a future therapy for neurodegenerative diseases : theme: drug discovery, development and delivery in Alzheimer’s disease guest editor: Davide Brambilla. Pharm Res 34(12):2614–2627. https://doi.org/10.1007/s11095-017-2199-y
Ertmer A, Gilch S, Yun SW, Flechsig E, Klebl B, Stein-Gerlach M, Klein MA, Schatzl HM (2004) The tyrosine kinase inhibitor STI571 induces cellular clearance of PrPSc in prion-infected cells. J Biol Chem 279(40):41918–41927. https://doi.org/10.1074/jbc.M405652200
Mahal SP, Baker CA, Demczyk CA, Smith EW, Julius C, Weissmann C (2007) Prion strain discrimination in cell culture: the cell panel assay. Proc Natl Acad Sci U S A 104(52):20908–20913. https://doi.org/10.1073/pnas.0710054104
Gilch S, Winklhofer KF, Groschup MH, Nunziante M, Lucassen R, Spielhaupter C, Muranyi W, Riesner D et al (2001) Intracellular re-routing of prion protein prevents propagation of PrP(Sc) and delays onset of prion disease. EMBO J 20(15):3957–3966. https://doi.org/10.1093/emboj/20.15.3957
John TR, Schatzl HM, Gilch S (2013) Early detection of chronic wasting disease prions in urine of pre-symptomatic deer by real-time quaking-induced conversion assay. Prion 7(3):253–258. https://doi.org/10.4161/pri.24430
Acknowledgments
We would like to thank our animal care technicians, Dr. Lillian Oribhabor and Bukola Alli, for their excellent support in animal husbandry and care. We also would like to thank Lauren Vankuppeveld for her help in cell culture-related work. We are also thankful to Yo-Ching Cheng for preparing the recombinant mouse PrP substrate used in RT-QuIC assay.
Funding
This work was performed within the framework of the Calgary Prion Research Unit and was supported by grants from Alberta Innovates/Alberta Prion Research Institute (APRI grant 201600010). ST is a University of Calgary Eyes High, Killam doctoral, and Alberta Innovates Health Solution (AIHS)-Health doctoral fellow. DHA was a University of Calgary Eyes High postdoctoral fellow; BAA was an AIHS postdoctoral fellow.
Author information
Authors and Affiliations
Contributions
ST, DHA, and HMS designed the experiments. ST, DHA, and BAA conducted the experiments. ST and DHA performed the animal experiments. ST and HMS wrote the manuscript. The manuscript was revised and approved by all authors.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thapa, S., Abdelaziz, D.H., Abdulrahman, B.A. et al. Sephin1 Reduces Prion Infection in Prion-Infected Cells and Animal Model. Mol Neurobiol 57, 2206–2219 (2020). https://doi.org/10.1007/s12035-020-01880-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-01880-y