Abstract
Purpose
Primary tumor (PT) and metastatic lymph node (MLN) status have a great influence on diagnosis and treatment of lung cancer. Our main purpose was to investigate the imaging characteristics of PT or MLN by applying the 18F-FDG PET dynamic modeling approach for non-small cell lung cancer (NSCLC).
Methods
Dynamic 18F-FDG PET scans were performed for 76 lung cancer patients, and 62 NSCLC cases were finally included in this study: 37 with newly diagnosed early and locally advanced lung cancer without distant metastases (group M0) and 25 metastatic lung cancer (group M1). Patlak graphic analysis (Ki calculation) based on the dynamic modeling and SUV analysis from conventional static data were performed.
Results
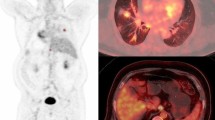
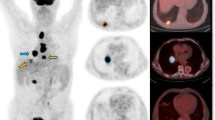
For PT, both KiPT (0.050 ± 0.005 vs 0.026 ± 0.004 min−1, p < 0.001) and SUVPT (8.41 ± 0.64 vs 5.23 ± 0.73, p < 0.01) showed significant higher values in group M1 than M0. For MLN, KiMLN showed significant higher values in M1 than M0 (0.033 ± 0.005 vs 0.016 ± 0.003 min−1, p < 0.01), while no significant differences were found for SUVMLN between M0 and M1 (4.22 ± 0.49 vs 5.57 ± 0.59, p > 0.05). Both SUV PT and KiPT showed significant high values in squamous cell carcinoma than adenocarcinoma, but neither SUVPT nor KiPT showed significant differences between EGFR mutants versus wild types. The overall Spearman analysis for SUV and Ki from different groups showed variable correlation (r = 0.46–0.94).
Conclusion
The dynamic modeling for MLN (KiMLN) showed more sensitive than the static analysis (SUV) to detect metastatic lymph nodes in NSCLC, although both methods were sensitive for PT. This methodology of non-invasive imaging may become an important tool to evaluate MLN and PT status for patients who cannot undergo histological examination.
Clinical trial registration
The clinical trial registration number is NCT03679936 (http://www.clinicaltrials.gov/).







Similar content being viewed by others
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
May M. Statistics: attacking an epidemic. Nature. 2014;509:S50–1. https://doi.org/10.1038/509S50a.
Lortet-Tieulent J, Soerjomataram I, Ferlay J, Rutherford M, Weiderpass E, Bray F. International trends in lung cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer (Amsterdam, Netherlands). 2014;84:13–22. https://doi.org/10.1016/j.lungcan.2014.01.009.
Strauss LG. Fluorine-18 deoxyglucose and false-positive results: a major problem in the diagnostics of oncological patients. Eur J Nucl Med. 1996;23:1409–15.
Som P, Atkins HL, Bandoypadhyay D, Fowler JS, MacGregor RR, Matsui K, et al. A fluorinated glucose analog, 2-fluoro-2-deoxy-D-glucose (F-18): nontoxic tracer for rapid tumor detection. J Nucl Med : official publication, Society of Nuclear Medicine. 1980;21:670–5.
Hamberg LM, Hunter GJ, Alpert NM, Choi NC, Babich JW, Fischman AJ. The dose uptake ratio as an index of glucose metabolism: useful parameter or oversimplification? J Nucl Med : official publication, Society of Nuclear Medicine. 1994;35:1308–12.
Huang SC. Anatomy of SUV. Standardized uptake value. Nucl Med Biol. 2000;27:643–6.
Keyes JW Jr. SUV: standard uptake or silly useless value? J Nucl Med : official publication, Society of Nuclear Medicine. 1995;36:1836–9.
Sugawara Y, Zasadny KR, Neuhoff AW, Wahl RL. Reevaluation of the standardized uptake value for FDG: variations with body weight and methods for correction. Radiology. 1999;213:521–5. https://doi.org/10.1148/radiology.213.2.r99nv37521.
Zasadny KR, Wahl RL. Standardized uptake values of normal tissues at PET with 2-[fluorine-18]-fluoro-2-deoxy-D-glucose: variations with body weight and a method for correction. Radiology. 1993;189:847–50. https://doi.org/10.1148/radiology.189.3.8234714.
Laffon E, de Clermont H, Begueret H, Vernejoux JM, Thumerel M, Marthan R, et al. Assessment of dual-time-point 18F-FDG-PET imaging for pulmonary lesions. Nucl Med Commun. 2009;30:455–61. https://doi.org/10.1097/MNM.0b013e32832bdcac.
Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1983;3:1–7. https://doi.org/10.1038/jcbfm.1983.1.
Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: validation of method. Ann Neurol. 1979;6:371–88. https://doi.org/10.1002/ana.410060502.
Gambhir SS, Schwaiger M, Huang SC, Krivokapich J, Schelbert HR, Nienaber CA, et al. Simple noninvasive quantification method for measuring myocardial glucose utilization in humans employing positron emission tomography and fluorine-18 deoxyglucose. J Nucl Med : official publication, Society of Nuclear Medicine. 1989;30:359–66.
Chen K, Bandy D, Reiman E, Huang SC, Lawson M, Feng D, et al. Noninvasive quantification of the cerebral metabolic rate for glucose using positron emission tomography, 18F-fluoro-2-deoxyglucose, the Patlak method, and an image-derived input function. J Cereb Blood Flow Metab : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1998;18:716–23. https://doi.org/10.1097/00004647-199807000-00002.
van der Weerdt AP, Klein LJ, Boellaard R, Visser CA, Visser FC, Lammertsma AA. Image-derived input functions for determination of MRGlu in cardiac (18)F-FDG PET scans. J Nucl Med : official publication, Society of Nuclear Medicine. 2001;42:1622–9.
Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest. 2017;151:193–203. https://doi.org/10.1016/j.chest.2016.10.010.
Boellaard R. Standards for PET image acquisition and quantitative data analysis. J Nucl Med : official publication, Society of Nuclear Medicine. 2009;50(Suppl 1):11s–20s. https://doi.org/10.2967/jnumed.108.057182.
Boellaard R, Oyen WJ, Hoekstra CJ, Hoekstra OS, Visser EP, Willemsen AT, et al. The Netherlands protocol for standardisation and quantification of FDG whole body PET studies in multi-centre trials. Eur J Nucl Med Mol Imaging. 2008;35:2320–33. https://doi.org/10.1007/s00259-008-0874-2.
Boellaard R, van Lingen A, Lammertsma AA. Experimental and clinical evaluation of iterative reconstruction (OSEM) in dynamic PET: quantitative characteristics and effects on kinetic modeling. J Nucl Med : official publication, Society of Nuclear Medicine. 2001;42:808–17.
Hudson HM, Larkin RS. Accelerated image reconstruction using ordered subsets of projection data. IEEE Trans Med Imaging. 1994;13:601–9. https://doi.org/10.1109/42.363108.
Freedman NM, Sundaram SK, Kurdziel K, Carrasquillo JA, Whatley M, Carson JM, et al. Comparison of SUV and Patlak slope for monitoring of cancer therapy using serial PET scans. Eur J Nucl Med Mol Imaging. 2003;30:46–53. https://doi.org/10.1007/s00259-002-0981-4.
Vieira S, Corrente JE. Statistical methods for assessing agreement between double readings of clinical measurements. J Appl Oral Sci : revista FOB. 2011;19:488–92.
van Baardwijk A, Dooms C, van Suylen RJ, Verbeken E, Hochstenbag M, Dehing-Oberije C, et al. The maximum uptake of (18)F-deoxyglucose on positron emission tomography scan correlates with survival, hypoxia inducible factor-1alpha and GLUT-1 in non-small cell lung cancer. Eur J Cancer (Oxford, England : 1990). 2007;43:1392–8. https://doi.org/10.1016/j.ejca.2007.03.027.
Chung JK, Lee YJ, Kim SK, Jeong JM, Lee DS, Lee MC. Comparison of [18F]fluorodeoxyglucose uptake with glucose transporter-1 expression and proliferation rate in human glioma and non-small-cell lung cancer. Nucl Med Commun. 2004;25:11–7.
Higashi K, Ueda Y, Sakurai A, Mingwang X, Xu L, Murakami M, et al. Correlation of Glut-1 glucose transporter expression with [(18)F]FDG uptake in non-small cell lung cancer. Eur J Nucl Med. 2000;27:1778–85. https://doi.org/10.1007/s002590000367.
van Berkel A, Vriens D, Visser EP, Janssen MJR, Gotthardt M, Hermus A, et al. Metabolic subtyping of pheochromocytoma and paraganglioma by (18)F-FDG pharmacokinetics using dynamic PET/CT scanning. J Nucl Med : official publication, Society of Nuclear Medicine. 2019;60:745–51. https://doi.org/10.2967/jnumed.118.216796.
de Geus-Oei LF, van Krieken JH, Aliredjo RP, Krabbe PF, Frielink C, Verhagen AF, et al. Biological correlates of FDG uptake in non-small cell lung cancer. Lung Cancer (Amsterdam, Netherlands). 2007;55:79–87. https://doi.org/10.1016/j.lungcan.2006.08.018.
Weber WA, Ziegler SI, Thodtmann R, Hanauske AR, Schwaiger M. Reproducibility of metabolic measurements in malignant tumors using FDG PET. J Nucl Med : official publication, Society of Nuclear Medicine. 1999;40:1771–7.
Minn H, Leskinen-Kallio S, Lindholm P, Bergman J, Ruotsalainen U, Teras M, et al. [18F]fluorodeoxyglucose uptake in tumors: kinetic vs. steady-state methods with reference to plasma insulin. J Comput Assist Tomogr. 1993;17:115–23.
Minn H, Zasadny KR, Quint LE, Wahl RL. Lung cancer: reproducibility of quantitative measurements for evaluating 2-[F-18]-fluoro-2-deoxy-D-glucose uptake at PET. Radiology. 1995;196:167–73. https://doi.org/10.1148/radiology.196.1.7784562.
Lodge MA, Lucas JD, Marsden PK, Cronin BF, O'Doherty MJ, Smith MA. A PET study of 18FDG uptake in soft tissue masses. Eur J Nucl Med. 1999;26:22–30.
Gallagher BM, Fowler JS, Gutterson NI, MacGregor RR, Wan CN, Wolf AP. Metabolic trapping as a principle of oradiopharmaceutical design: some factors resposible for the biodistribution of [18F] 2-deoxy-2-fluoro-D-glucose. J Nucl Med : official publication, Society of Nuclear Medicine. 1978;19:1154–61.
Hoekstra CJ, Paglianiti I, Hoekstra OS, Smit EF, Postmus PE, Teule GJ, et al. Monitoring response to therapy in cancer using [18F]-2-fluoro-2-deoxy-D-glucose and positron emission tomography: an overview of different analytical methods. Eur J Nucl Med. 2000;27:731–43.
Sundaram SK, Freedman NM, Carrasquillo JA, Carson JM, Whatley M, Libutti SK, et al. Simplified kinetic analysis of tumor 18F-FDG uptake: a dynamic approach. J Nucl Med : official publication, Society of Nuclear Medicine. 2004;45:1328–33.
McDermott GM, Welch A, Staff RT, Gilbert FJ, Schweiger L, Semple SI, et al. Monitoring primary breast cancer throughout chemotherapy using FDG-PET. Breast Cancer Res Treat. 2007;102:75–84. https://doi.org/10.1007/s10549-006-9316-7.
Sun X, Xiao Z, Chen G, Han Z, Liu Y, Zhang C, et al. A PET imaging approach for determining EGFR mutation status for improved lung cancer patient management. Sci Transl Med. 2018;10. https://doi.org/10.1126/scitranslmed.aan8840.
Choi YJ, Cho BC, Jeong YH, Seo HJ, Kim HJ, Cho A, et al. Correlation between (18)f-fluorodeoxyglucose uptake and epidermal growth factor receptor mutations in advanced lung cancer. Nucl Med Mol Imaging. 2012;46:169–75. https://doi.org/10.1007/s13139-012-0142-z.
Huang CT, Yen RF, Cheng MF, Hsu YC, Wei PF, Tsai YJ, et al. Correlation of F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value and EGFR mutations in advanced lung adenocarcinoma. Medical Oncology (Northwood, London, England). 2010;27:9–15. https://doi.org/10.1007/s12032-008-9160-1.
Funding
This work was funded by the National Key R&D Program of China (2018YFC0910601), the National Natural Science Foundation of China (No.81871382), and Starting Fund from Sun Yat-sen University Fifth Affiliated Hospital.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design; the final analysis and writing of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology – Chest.
Electronic supplementary material
ESM. 1
(JPG 38 kb)
Rights and permissions
About this article
Cite this article
Yang, M., Lin, Z., Xu, Z. et al. Influx rate constant of 18F-FDG increases in metastatic lymph nodes of non-small cell lung cancer patients. Eur J Nucl Med Mol Imaging 47, 1198–1208 (2020). https://doi.org/10.1007/s00259-020-04682-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-04682-5