Abstract
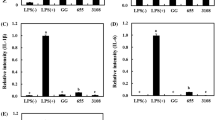
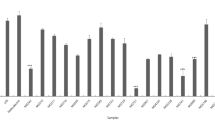
The aim of this study was to assess the protective effect of the intracellular content obtained from potential probiotic bacteria against acrylamide-induced oxidative damage in human erythrocytes. First, the antioxidant properties of 12 potential probiotic strains was evaluated. Two commercial probiotic bacteria were included as reference strains, namely, Lactobacillus casei Shirota and Lactobacillus paracasei 431. Data showed that the intracellular content from four strains, i.e., Lactobacillus fermentum J10, Lactobacillus pentosus J24 and J26, and Lactobacillus pentosus J27, showed higher (P < 0.05) antioxidant capacity in most methods used. Thereafter, the intracellular content of such pre-selected strains was able to prevent the disturbance of the antioxidant system of human erythrocytes exposed to acrylamide, thereby reducing cell disruption and eryptosis development (P < 0.05). Additionally, the degree of oxidative stress in erythrocytes exposed to acrylamide was significantly (P < 0.05) reduced to levels similar to the basal conditions when the intracellular content of Lact. fermentum J10, Lact. pentosus J27, and Lact. paracasei 431 were employed. Hence, our findings suggest that the intracellular contents of specific Lactobacillus strains represent a potential source of metabolites with antioxidant properties that may help reduce the oxidative stress induced by acrylamide in human erythrocytes.





Similar content being viewed by others
References
van Boekel M, Fogliano V, Pellegrini N, Stanton C, Scholz G, Lalljie S, Somoza V, Knorr D, Jasti PR, Eisenbrand G (2010) A review on the beneficial aspects of food processing. Mol Nutr Food Res 54:1215–1247. https://doi.org/10.1002/mnfr.200900608
EFSA (2017) Commission regulation (EU) 2017/2158 of 20 November 2017 establishing mitigation measures and benchmark levels for the reduction of the presence of acrylamide in food. https://www.fsai.ie/uploadedFiles/Reg2017_2158.pdf. Accessed 30 July 2019
Capuano E, Fogliano V (2011) Acrylamide and 5-hydroxymethylfurfural (HMF): a review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT-Food Sci Technol 44:793–810. https://doi.org/10.1016/j.lwt.2010.11.002
Rivas-Jimenez L, Ramírez-Ortiz K, González-Córdova AF, Vallejo-Cordoba B, Garcia HS, Hernandez-Mendoza A (2016) Evaluation of acrylamide-removing properties of two Lactobacillus strains under simulated gastrointestinal conditions using a dynamic system. Microbiol Res 190:19–26. https://doi.org/10.1016/j.micres.2016.04.016
Zamani E, Shokrzadeh M, Fallah M, Shaki F (2017) A review of acrylamide toxicity and its mechanism. Pharm Biomed Res 3:1–7. https://doi.org/10.18869/acadpub.pbr.3.1.1
IARC (2018) Agents classified by the IARC monographs, volumes 1–123. Monographs on the identification of carcinogenic hazards to humans. https://monographs.iarc.fr/list-of-classifications-volumes/. Accessed 30 July 2019
Catalgol B, Ozhan G, Alpertunga B (2009) Acrylamide-induced oxidative stress in human erythrocytes. Hum Exp Toxicol 28:611–617. https://doi.org/10.1177/0960327109350664
Ghorbel I, Maktouf S, Kallel C, Ellouze Chaabouni S, Boudawara T, Zeghal N (2015) Disruption of erythrocyte antioxidant defense system, hematological parameters, induction of pro-inflammatory cytokines and DNA damage in liver of co-exposed rats to aluminium and acrylamide. Chem Biol Interact 236:31–40. https://doi.org/10.1016/j.cbi.2015.04.020
Kim K, Bae ON, Koh SH, Kang S, Lim KM, Noh JY, Shin S, Kim I, Chung JH (2015) High-dose vitamin c injection to cancer patients may promote thrombosis through procoagulant activation of erythrocytes. Toxicol Sci 147:350–359. https://doi.org/10.1093/toxsci/kfv133
Minneci PC, Deans KJ, Zhi H, Yuen PST, Star RA, Banks SM, Schechter AN, Natanson C, Gladwin MT, Solomon SB (2005) Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest 115:3409–3417. https://doi.org/10.1172/JCI25040.The
Ghorbel I, Chaabane M, Elwej A, Kallel C, Kamoun NG, Zeghal N (2017) Extra virgin olive oil mitigates hematotoxicity induced by acrylamide and oxidative damage in adult rats. Pharm Biomed Res 3:34–40
Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V (2012) Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 28:539–543. https://doi.org/10.1016/j.nut.2011.08.013
Hajifaraji M, Jahanjou F, Abbasalizadeh F, Aghamohammadzadeh N, Abbasi MM, Dolatkhah N (2018) Effect of probiotic supplements in women with gestational diabetes mellitus on inflammation and oxidative stress biomarkers: a randomized clinical trial. Asia Pac J Clin Nutr 27:581–591. https://doi.org/10.6133/apjcn.082017.03
Aguilar-Toalá JE, Estrada-Montoya MC, Liceaga AM, Garcia HS, González-Aguilar GA, Vallejo-Cordoba B, González-Córdova AF, Hernández-Mendoza A (2019) An insight on antioxidant properties of the intracellular content of Lactobacillus casei CRL-431. LWT-Food Sci Technol 102:58–63. https://doi.org/10.1016/j.lwt.2018.12.015
Li S, Huang R, Shah NP, Tao X, Xiong Y, Wei H (2014) Antioxidant and antibacterial activities of exopolysaccharides from Bifidobacterium bifidum WBIN03 and Lactobacillus plantarum R315. J Dairy Sci 97:7334–7343. https://doi.org/10.3168/jds.2014-7912
Shen Q, Zhang B, Xu R, Wang Y, Ding X, Li P (2010) Antioxidant activity in vitro of the selenium-contained protein from the Se-enriched Bifidobacterium animalis 01. Anaerobe 16:380–386. https://doi.org/10.1016/j.anaerobe.2010.06.006
Aguilar-Toalá JE, Santiago-López L, Peres CM, Peres C, Garcia HS, Vallejo-Cordoba B, González-Córdova AF, Hernández-Mendoza A (2017) Assessment of multifunctional activity of bioactive peptides derived from fermented milk by specific Lactobacillus plantarum strains. J Dairy Sci 100:65–75. https://doi.org/10.3168/jds.2016-11846
Ou CC, Chiu YH, Lin SL, Chang YJ, Huang HY, Lin MY (2012) Hepatoprotective effect of lactic acid bacteria in the attenuation of oxidative stress from tert-butyl hydroperoxide. J Food Drug Anal 20:101–110
Aguilar-Toalá JE, Hall FG, Urbizo-Reyes UC, Garcia HS, Vallejo-Cordoba B, González-Córdova AF, Hernández-Mendoza A, Liceaga AM (2019) In silico prediction and in vitro assessment of multifunctional properties of postbiotics obtained from two probiotic bacteria. Probiotics Antimicrob Proteins:1–15. https://doi.org/10.1007/s12602-019-09568-z
Wu D, Sun MZ, Zhang C, Xin Y (2014) Antioxidant properties of Lactobacillus and its protecting effects to oxidative stress Caco-2 cells. J Anim Plant Sci 24:1766–1771
Lin MY, Yen CL (1999) Inhibition of lipid peroxidation by Lactobacillus acidophilus and Bifidobacterium longum. J Agric Food Chem 47:3661–3664. https://doi.org/10.1021/jf981235l
Reyes-Díaz A, Mata-Haro V, Hernández J, Gonzalez-Cordova AF, Hernandez-Mendoza A, Reyes-Díaz R, Torres-Llanez MJ, Beltran-Barrientos LM, Vallejo-Córdoba B (2018) Milk fermented by specific Lactobacillus strains regulates the serum levels of IL-6, TNF- α and IL-10. Nutrients 10:1–15. https://doi.org/10.3390/nu10060691
Santiago-López L, Hernández-Mendoza A, Mata-Haro V, Vallejo-Cordoba B, González-Córdova AF (2018) Immune response induced by fermented milk with potential probiotic strains isolated from artisanal Cocido cheese. Food Agric Immunol 29:911–929. https://doi.org/10.1080/09540105.2018.1485632
Aguilar-Toalá JE, Astiazarán-García H, Estrada-Montoya MC, Garcia HS, Vallejo-Cordoba B, González-Córdova AF, Hernández-Mendoza A (2019) Modulatory effect of the intracellular content of Lactobacillus casei CRL 431 against the aflatoxin B1-induced oxidative stress in rats. Probiotics Antimicrob Proteins 11:470–477. https://doi.org/10.1007/s12602-018-9433-8
Herald TJ, Gadgil P, Tilley M (2012) High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J Sci Food Agric 92:2326–2331. https://doi.org/10.1002/jsfa.5633
Zulueta A, Esteve MJ, Frígola A (2009) ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem 114:310–316. https://doi.org/10.1016/j.foodchem.2008.09.033
Thilakarathna SH, Rupasinghe HPV, Needs PW (2013) Apple peel bioactive rich extracts effectively inhibit in vitro human LDL cholesterol oxidation. Food Chem 138:463–470. https://doi.org/10.1016/j.foodchem.2012.09.121
Serrano-Niño JC, Cavazos-Garduño A, Cantú-Cornelio F, González-Córdova AF, Vallejo-Córdoba B, Hernández-Mendoza A, García HS (2015) In vitro reduced availability of aflatoxin B1 and acrylamide by bonding interactions with teichoic acids from Lactobacillus strains. LWT-Food Sci Technol 64:1334–1341. https://doi.org/10.1016/j.lwt.2015.07.015
Qasim N, Mahmood R (2015) Diminution of oxidative damage to human erythrocytes and lymphocytes by creatine: possible role of creatine in blood. PLoS PNE 10:1–21. https://doi.org/10.1371/journal.pone.0141975
Meng F, Alayash AI (2017) Determination of extinction coefficients of human haemoglobin in various redox states. Anal Biochem 521:11–19. https://doi.org/10.1016/j.ab.2017.01.002
Aebi H (1984) [13] Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287. https://doi.org/10.1016/0003-2697(71)90370-8
Flohé L, Günzler WA (1984) [12] Assays of glutathione peroxidase. Methods Enzymol 105:114–120. https://doi.org/10.1016/S0076-6879(84)05015-1
Sinha M, Manna P, Sil PC (2007) A 43 kD protein from the herb, Cajanus indicus L., protects against fluoride induced oxidative stress in mice erythrocytes. Pathophysiology 14:47–54. https://doi.org/10.1016/j.pathophys.2007.01.001
Caetano AC, da Veiga LF, Capaldi FR, de Alencar SM, Azevedo RA, Bezerra RMN (2013) The antioxidant response of the liver of male Swiss mice raised on a AIN 93 or commercial diet. BMC Physiol 13:3. https://doi.org/10.1186/1472-6793-13-3
Zhang S, Liu L, Su Y, Li H, Sun Q, Liang X, Lv J (2011) Antioxidative activity of lactic acid bacteria in yogurt. Afr J Microbiol Res 5:5194–5201
Pieniz S, Andreazza R, Anghinoni T, Camargo F, Brandelli A (2014) Probiotic potential, antimicrobial and antioxidant activities of Enterococcus durans strain LAB18s. Food Control 37:251–256. https://doi.org/10.1016/j.foodcont.2013.09.055
Li S, Zhao Y, Zhang L, Zhang X, Huang L, Li D, Niu C, Yang Z, Wang Q (2012) Antioxidant activity of Lactobacillus plantarum strains isolated from traditional Chinese fermented foods. Food Chem 135:1914–1919. https://doi.org/10.1016/j.foodchem.2012.06.048
Amaretti A, Di Nunzio M, Pompei A, Raimondi S, Rossi M, Bordoni A (2013) Antioxidant properties of potentially probiotic bacteria: in vitro and in vivo activities. Appl Microbiol Biotechnol 97:809–817. https://doi.org/10.1007/s00253-012-4241-7
Kim HS, Chae HS, Jeong SG, Ham JS, Im SK, Ahn CN, Lee JM (2006) In vitro antioxidative properties of lactobacilli. Asian-Australas J Anim Sci 19:262–265
Lee J, Hwang KT, Chung MY, Cho DH, Park CS (2005) Resistance of Lactobacillus casei KCTC 3260 to reactive oxygen species (ROS): role for a metal ion chelating effect. J Food Sci 70:m388–m391. https://doi.org/10.1111/j.1365-2621.2005.tb11524.x
Ghiselli A, Serafini M, Natella F, Scaccini C (2000) Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radic Biol Med 29:1106–1114. https://doi.org/10.1016/S0891-5849(00)00394-4
Dixit R, Das M, Seth PK, Mukthar H (1984) Interaction of acrylamide with glutathione in rat erythrocytes. Toxicol Lett 23:291–298
Abuelo A, Hernández J, Benedito JL, Castillo C (2013) Oxidative stress index (OSi) as a new tool to assess redox status in dairy cattle during the transition period. Animal 7:1374–1378. https://doi.org/10.1017/S1751731113000396
Forman HJ, Zhang H, Rinna A (2010) Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Asp Med 30:1–12. https://doi.org/10.1016/j.mam.2008.08.006.Glutathione
Farag MR, Alagawany M (2018) Erythrocytes as a biological model for screening of xenobiotics toxicity. Chem Biol Interact 279:73–83. https://doi.org/10.1016/j.cbi.2017.11.007
Richter C (1987) Biophysical consequences of lipid peroxidation in membranes. Chem Phys Lipids 44:175–189. https://doi.org/10.1016/0009-3084(87)90049-1
Malinowski DP, Fridovich I (1979) Subunit association and side-chain reactivities of bovine erythrocyte superoxide dismutase in denaturing solvents. Biochemistry 18:5055–5060
Malinowski DP, Fridovich I (1979) Chemical modification of arginine at the active site of the bovine erythrocyte superoxide dismutase. Biochemistry 18:5909–5917
Tesoriere L, Allegra M, Butera D, Gentile C, Livrea MA (2006) Cytoprotective effects of the antioxidant phytochemical indicaxanthin in β-thalassemia red blood cells. Free Radic Res 40:753–761. https://doi.org/10.1080/10715760600554228
Begum N, Terao J (2003) Protective effect of α-tocotrienol against free radical-induced impairment of erythrocyte deformability. Biosci Biotechnol Biochem 66:398–403. https://doi.org/10.1271/bbb.66.398
Chaudhuri S, Banerjee A, Basu K, Sengupta B, Sengupta PK (2007) Interaction of flavonoids with red blood cell membrane lipids and proteins: antioxidant and antihemolytic effects. Int J Biol Macromol 41:42–48. https://doi.org/10.1016/j.ijbiomac.2006.12.003
Chauhan R, Sudhakaran VA, Panwar H, Mallapa RH, Duary RK, Batish VK, Grover S (2014) Amelioration of colitis in mouse model by exploring antioxidative potentials of an indigenous probiotic strain of Lactobacillus fermentum Lf1. Biomed Res Int 2014. https://doi.org/10.1155/2014/206732
Jones RM, Desai C, Darby TM, Luo L, Wolfarth AA, Scharer CD, Ardita CS, Reedy AR, Keebaugh ES, Neish AS (2015) Lactobacilli modulate epithelial cytoprotection through the Nrf2 pathway. Cell Rep 12:1217–1225. https://doi.org/10.1016/j.celrep.2015.07.042
Endo H, Niioka M, Kobayashi N, Tanaka M, Watanabe T (2013) Butyrate-producing probiotics reduce nonalcoholic fatty liver disease progression in rats: new insight into the probiotics for the gut-liver axis. PLoS One 8:e63388. https://doi.org/10.1371/journal.pone.0063388
Acknowledgments
P. F. Cuevas-González was supported by a graduate scholarship from the National Council for Science and Technology (CONACyT) of Mexico. The authors are grateful for the technical support of J.N. González-González and L. Santiago-López.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Institutional Ethics Committee of Centro de Investigación en Alimentación y Desarrollo, A.C., CE/007/2019) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author Contributions
A. Hernández-Mendoza contributed to the study conception and design. Material preparation, data collection, and analysis were performed by P.F. Cuevas-González and J.E. Aguilar-Toalá. The first draft of the manuscript was written by P.F. Cuevas-González, and all the authors edited and commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Rights and permissions
About this article
Cite this article
Cuevas-González, P., Aguilar-Toalá, J., García, H. et al. Protective Effect of the Intracellular Content from Potential Probiotic Bacteria against Oxidative Damage Induced by Acrylamide in Human Erythrocytes. Probiotics & Antimicro. Prot. 12, 1459–1470 (2020). https://doi.org/10.1007/s12602-020-09636-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-020-09636-9