Abstract
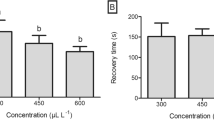
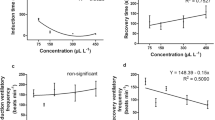
The efficacy of three common fish anesthetics (clove oil, 2-phenoxyethanol, and tricaine methanesulfonate) was evaluated in the Pacific hagfish (Eptatretus stoutii). The overarching aim of our study was to identify the best anesthetic and concentration for the purposes of routine laboratory use of Pacific hagfish (i.e., short and consistent induction and recovery times and minimized stress and safety risk to hagfish). The objectives of our study were fourfold: (1) identify anesthetic stages of Pacific hagfish using clove oil anesthesia; (2) establish standardized anesthesia preparation procedures; (3) determine the optimal anesthetic and concentration for safely achieving stage V anesthesia; and (4) investigate the effects of repeatedly exposing Pacific hagfish to anesthesia. Experimental concentrations, ranging from 50 to 400 mg/L, of each anesthetic were tested on at least three Pacific hagfish individuals. We found the following: (1) Pacific hagfish exhibited similar stages of anesthesia to those described for bony fishes; (2) sufficient mixing of clove oil with seawater had a considerable effect on the consistency and timing of anesthetic induction; (3) concentration and anesthetic significantly impacted induction and recovery timing, whereas body mass had no impact on anesthetic trends; and (4) repeatedly exposing Pacific hagfish to optimal concentrations of clove oil or MS-222 had no effect on induction or recovery timing, whereas exposure number significantly impacted induction timing when using 2-PE. Due to consistent induction and recovery times, low risk of accidental overdose, and high safety margins for both handler and hagfish, we recommend 175 mg/L of clove oil as the ideal anesthetic and concentration for the routine laboratory use of Pacific hagfish.








Similar content being viewed by others
References
Bernstein PS, Digre KB, Creel DJ (1997) Retinal Toxicity Associated With Occupational Exposure to the Fish Anesthetic MS–222. American Journal of Ophthalmology 124(6):843–844
Bolasina SN (2006) Cortisol and hematological response in Brazilian codling, Urophycis brasiliensis (Pisces, Phycidae) subjected to anesthetic treatment. Aquac Int 14(6):569–575
Böni L, Rühs PA, Windhab EJ, Fischer P, Kuster S (2016) Gelation of soy milk with hagfish exudate creates a flocculated and fibrous emulsion-and particle gel. PLoS One 11(1):e0147022
Böni LJ, Sanchez-Ferrer A, Widmer M, Biviano MD, Mezzenga R, Windhab EJ, Dagastine RR, Fischer P (2018) Structure and nanomechanics of dry and hydrated intermediate filament films and fibers produced from hagfish slime fibers. ACS Appl Mater Interfaces 10(47):40460–40473
Burka JF, Hammell KL, Horsberg TE, Johnson GR, Rainnie DJ, Speare DJ (1997) Drugs in salmonid aquaculture–a review. J Vet Pharmacol Ther 20(5):333–349
Carter KM, Woodley CM, Brown RS (2011) A review of tricaine methanesulfonate for anesthesia of fish. Rev Fish Biol Fish 21(1):51–59
Clifford AM, Bury NR, Schultz AG, Ede JD, Goss BL, Goss GG (2017) Regulation of plasma glucose and sulfate excretion in Pacific hagfish, Eptatretus stoutii is not mediated by 11-deoxycortisol. Gen Comp Endocrinol 247:107–115
Downing SW, Salo WL, Spitzer RH, Koch EA (1981) The hagfish slime gland: a model system for studying the biology of mucus. Science 214(4525):1143–1145
FDA (US Food and Drug Administration) (2006) Database of approved animal drug products. US Department of Health and Human Services, Food and Drug Administration, Center for Veterinary Medicine, Rockville
Fischer IU, Von Unruh GE, Dengler HJ (1990) The metabolism of eugenol in man. Xenobiotica 20(2):209–222
Flavor and Extract Manufacturers' Association. National Technical Information Service, US Department of Commerce (1978) Scientific Literature Review of Eugenol and Related Substances in Flavor Usage (3 edn.), Accession No. PB-283 501, Vol. I
Foster JM, Forster ME (2007) Changes in plasma catecholamine concentration during salinity manipulation and anaesthesia in the hagfish Eptatretus cirrhatus. J Comp Physiol B 177(1):41–47
Fudge DS, Gardner KH, Forsyth VT, Riekel C, Gosline JM (2003) The mechanical properties of hydrated intermediate filaments: insights from hagfish slime threads. Biophys J 85(3):2015–2027
Fudge DS, Levy N, Chiu S, Gosline JM (2005) Composition, morphology and mechanics of hagfish slime. J Exp Biol 208(24):4613–4625
Fudge DS, Hillis S, Levy N, Gosline JM (2010) Hagfish slime threads as a biomimetic model for high performance protein fibres. Bioinspir Biomim 5(3):035002
Germain P, Gagnon A (1968) A simple and reliable method for obtaining large blood samples from the anaesthetized hagfish. Comp Biochem Physiol 26(1):371–375
Gilderhus PA, Marking LL (1987) Comparative efficacy of 16 anesthetic chemicals on rainbow trout. N Am J Fish Manag 7(2):288–292
Hoskonen P, Pirhonen J (2004) Temperature effects on anaesthesia with clove oil in six temperate-zone fishes. J Fish Biol 64(4):1136–1142
Houston AH, Corlett JT, Woods RJ (1976) Specimen weight and MS 222. Journal of the Fisheries Board of Canada 33(6):1403–1407
Javahery S, Nekoubin H, Moradlu AH (2012) Effect of anaesthesia with clove oil in fish. Fish Physiol Biochem 38(6):1545–1552
Keene JL, Noakes DLG, Moccia RD, Soto CG (1998) The efficacy of clove oil as an anaesthetic for rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac Res 29(2):89–101
King W, Hooper B, Hillsgrove S, Benton C, Berlinsky DL (2005) The use of clove oil, metomidate, tricaine methanesulphonate and 2-phenoxyethanol for inducing anaesthesia and their effect on the cortisol stress response in black sea bass (Centropristis striata L.). Aquac Res 36(14):1442–1449
Lim J, Fudge DS, Levy N, Gosline JM (2006) Hagfish slime ecomechanics: testing the gill-clogging hypothesis. J Exp Biol 209(4):702–710
Matthews M, Varga ZM (2012) Anesthesia and euthanasia in zebrafish. ILAR J 53(2):192–204
Mitjana O, Bonastre C, Insua D, Falceto MV, Esteban J, Josa A, Espinosa E (2014) The efficacy and effect of repeated exposure to 2-phenoxyethanol, clove oil and tricaine methanesulphonate as anesthetic agents on juvenile Angelfish (Pterophyllum scalare). Aquaculture 433:491–495
Molinero A, Gonzalez J (1995) Comparative effects of MS 222 and 2-phenoxyethanol on gilthead sea bream (Sparus aurata L.) during confinement. Comp Biochem Physiol A Physiol 111(3):405–414
Munday PL, Wilson SK (1997) Comparative efficacy of clove oil and other chemicals in anaesthetization of Pomacentrus amboinensis, a coral reef fish. J Fish Biol 51(5):931–938
Murray MJ (2002) Fish surgery. In: Seminars in avian and exotic pet medicine, vol 11, No. 4. WB Saunders, Philadelphia, pp 246–257
Mylonas CC, Cardinaletti G, Sigelaki I, Polzonetti-Magni A (2005) Comparative efficacy of clove oil and 2-phenoxyethanol as anesthetics in the aquaculture of European sea bass (Dicentrarchus labrax) and gilthead sea bream (Sparus aurata) at different temperatures. Aquaculture 246(1–4):467–481
Neiffer DL, Stamper MA (2009) Fish sedation, anesthesia, analgesia, and euthanasia: considerations, methods, and types of drugs. ILAR J 50(4):343–360
Pawar HB, Sanaye SV, Sreepada RA, Harish V, Suryavanshi U, Ansari ZA (2011) Comparative efficacy of four anaesthetic agents in the yellow seahorse, Hippocampus kuda (Bleeker, 1852). Aquaculture 311(1–4):155–161
Priborsky J, Velisek J (2018) A review of three commonly used fish anesthetics. Rev Fish Sci Aquac 26(4):417–442
Rodríguez-Gutiérrez M, Esquivel-Herrera A (1995) Evaluation of the repeated use of xylocaine as anesthetic for the handling of breeding carp (Cyprinus carpio). Aquaculture 129(1–4):431–436
Schorno S, Gillis TE, Fudge DS (2018) Cellular mechanisms of slime gland refilling in Pacific hagfish (Eptatretus stoutii). J Exp Biol 221(16):jeb183806
Shaluei F, Hedayati A, Jahanbakhshi A, Baghfalaki M (2012) Physiological responses of great sturgeon (Huso huso) to different concentrations of 2-phenoxyethanol as an anesthetic. Fish Physiol Biochem 38(6):1627–1634
Soto CG (1995) Clove oil as a fish anaesthetic for measuring length and weight of rabbitfish (Siganus lineatus). Aquaculture 136(1–2):149–152
Stoskopf MK (1985) Manual for the aquatic animal workshop. American Association for Laboratory Animal Science, National Capital Area Branch, Washington D.C.
Strange RJ, Schreck CB (1978) Anesthetic and handling stress on survival and cortisol concentration in yearling Chinook salmon (Oncorhynchus tshawytscha). J Fish Board Can 35(3):345–349
Topic Popovic N, Strunjak-Perovic I, Coz-Rakovac R, Barisic J, Jadan M, Persin Berakovic A, Sauerborn Klobucar R (2012) Tricaine methane-sulfonate (MS-222) application in fish anaesthesia. J Appl Ichthyol 28(4):553–564
Varkey T, Mercy A, Sajeevan S (2014) Efficacy of 2-phenoxyethanol as an anaesthetic for adult redline torpedo fish, Sahyadria denisonii (day 1865). Int J Zool: 1–4
Velíšek J, Stejskal V, Kouřil J, Svobodová Z (2009) Comparison of the effects of four anaesthetics on biochemical blood profiles of perch. Aquac Res 40(3):354–361
Waterstrat PR (1999) Induction and recovery from anesthesia in channel catfish Ictalurus punctatus fingerlings exposed to clove oil. J World Aquacult Soc 30(2):250–255
Weber RA, Peleteiro JB, Martín LG, Aldegunde M (2009) The efficacy of 2-phenoxyethanol, metomidate, clove oil and MS-222 as anaesthetic agents in the Senegalese sole (Solea senegalensis Kaup 1858). Aquaculture 288(1–2):147–150
Wedemeyer G (1970) Stress of anesthesia with MS 222 and benzocaine in rainbow trout (Salmo gairdneri). J Fish Board Can 27(5):909–914
Weyl O, Kaiser H, Hecht T (1996) On the efficacy and mode of action of 2-phenoxyethanol as an anaesthetic for goldfish, Carassius auratus (L.), at different temperatures and concentrations. Aquac Res 27(10):757–764
Winegard TM, Fudge DS (2010) Deployment of hagfish slime thread skeins requires the transmission of mixing forces via mucin strands. J Exp Biol 213(8):1235–1240
Zintzen V, Roberts CD, Anderson MJ, Stewart AL, Struthers CD, Harvey ES (2011) Hagfish predatory behaviour and slime defence mechanism. Sci Rep 1:131
Acknowledgments
The authors would like to thank the members of the Chapman University Comparative Biomaterials Lab and SURFEES Program for their constructive feedback on early drafts of this manuscript, data collection during pilot studies, and assistance in caring for the hagfish used in this study. We are also grateful to John Gregg and Jerid Rold for providing Pacific hagfish specimens to the Fudge Lab.
Funding
This work was supported by an NSF IOS grant to DF and Grand Challenges Initiative research support funds to CM.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
McCord, C.L., Whiteley, E., Liang, J. et al. Concentration effects of three common fish anesthetics on Pacific hagfish (Eptatretus stoutii). Fish Physiol Biochem 46, 931–943 (2020). https://doi.org/10.1007/s10695-020-00761-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-020-00761-4