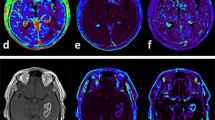
Abstract
Purpose
To examine the applicability of contrast leakage information from dynamic susceptibility contrast-enhanced (DSC) MRI and dynamic contrast-enhanced (DCE) MRI to determine which one is the most valuable surrogate imaging biomarker for predicting disease progression in anaplastic astrocytoma (AA) patients.
Materials and methods
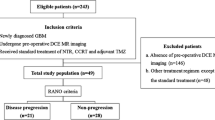
This study was approved by the institutional review board (IRB), which waived informed consent. A total of seventy-three AA patients who had undergone preoperative DCE and DSC MRI and received standard treatment, including partial resection or biopsy followed by radiation therapy, were included in this retrospective study. Based on Response Assessment in Neuro-Oncology (RANO), patients were sorted into progression (n = 21) and non-progression (n = 52) groups. Tumor boundaries were defined as high-signal intensity (SI) lesions on fluid-attenuated inversion recovery (FLAIR) imaging, where we analyzed mean pharmacokinetic parameters (Ktrans, Vp, and Ve) from DCE MRI and contrast leakage information (mean extraction fraction (EF)) from DSC MRI.
Results
Mean Ve and mean EF were significantly higher in patients with progression-free survival (PFS) < 18 months than in those with PFS ≥ 18 months. For distinguishing the group with PFS < 18 months, AUC values were calculated using the mean Ve value (AUC = 0.716). The Kaplan-Meier survival analysis revealed that mean Ve value was significantly correlated with PFS. In Cox proportional-hazards regression, only the mean Ve value was found to be significantly associated with PFS.
Conclusion
We found that the mean Ve value based on high-SI tumor lesions on FLAIR imaging was capable of predicting outcomes of AA patients as a potential surrogate imaging biomarker.
Key Points
• Mean Ve(2.152 ± 1.857 vs. 1.173 ± 1.408) was significantly higher in anaplastic astrocytoma patients with PFS < 18 months that in those with PFS ≥ 18 months (p = 0.02).
• Cox proportional-hazards regression showed that only mean Ve(p = 0.034) was significantly associated with PFS, regardless of IDH mutation status, in anaplastic astrocytoma patients.




Similar content being viewed by others
Abbreviations
- AA:
-
Anaplastic astrocytoma
- AIF:
-
Arterial input function
- AUC:
-
Area under the receiver operating characteristic curve
- BBB:
-
Blood-brain barrier
- DCE:
-
Dynamic contrast-enhanced
- DSC:
-
Dynamic susceptibility contrast-enhanced
- EES:
-
Extravascular extracellular space
- EF:
-
Extraction fraction, contrast leakage information of DSC MRI
- FLAIR:
-
Fluid-attenuated inversion recovery
- FOV:
-
Field of view
- IDH:
-
Isocitrate dehydrogenase
- K trans :
-
Volume transfer constant
- MPRAGE:
-
Magnetization-prepared rapid acquisition gradient-echo
- NEX:
-
Number of excitations
- PFS:
-
Progression-free survival
- RANO:
-
Response Assessment in Neuro-Oncology
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region of interest
- RT:
-
Radiotherapy
- SI:
-
Signal intensity
- T1WI:
-
T1-weighted imaging
- T2WI:
-
T2-weighted imaging
- TE:
-
Echo time
- TI:
-
Inversion time
- TR:
-
Repetition time
- V e :
-
Extravascular extracellular space volume per unit volume of tissue
- VOI:
-
Volume of interest
- V p :
-
Blood plasma volume per unit volume of tissue
- WHO:
-
World Health Organization
References
Louis DN, Ohgaki H, Wiestler OD et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Prados MD, Gutin PH, Phillips TL et al (1992) Highly anaplastic astrocytoma: a review of 357 patients treated between 1977 and 1989. Int J Radiat Oncol Biol Phys 23:3–8
Plate KH, Breier G, Weich HA, Risau W (1992) Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature 359:845
Jain R (2013) Measurements of tumor vascular leakiness using DCE in brain tumors: clinical applications. NMR Biomed 26:1042–1049
Heye AK, Culling RD, Valdés Hernández Mdel C, Thrippleton MJ, Wardlaw JM (2014) Assessment of blood–brain barrier disruption using dynamic contrast-enhanced MRI. A systematic review. Neuroimage Clin 6:262–274
Yoo R-E, Choi SH, Kim TM et al (2017) Dynamic contrast-enhanced MR imaging in predicting progression of enhancing lesions persisting after standard treatment in glioblastoma patients: a prospective study. Eur Radiol 27:3156–3166
Harrer JU, Parker GJ, Haroon HA et al (2004) Comparative study of methods for determining vascular permeability and blood volume in human gliomas. J Magn Reson Imaging 20:748–757
Tofts PS, Brix G, Buckley DL et al (1999) Estimating kinetic parameters from dynamic contrast-enhanced T1-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 10:223–232
Lee B, Park J, Bjørnerud A, Kim J, Lee J, Kim H (2018) Clinical value of vascular permeability estimates using dynamic susceptibility contrast MRI: improved diagnostic performance in distinguishing hypervascular primary CNS lymphoma from glioblastoma. AJNR Am J Neuroradiol 39:1415–1422
Ulyte A, Katsaros VK, Liouta E et al (2016) Prognostic value of preoperative dynamic contrast-enhanced MRI perfusion parameters for high-grade glioma patients. Neuroradiology 58:1197–1208
Nguyen TB, Cron GO, Mercier JF et al (2015) Preoperative prognostic value of dynamic contrast-enhanced MRI–derived contrast transfer coefficient and plasma volume in patients with cerebral gliomas. AJNR Am J Neuroradiol 36:63–69
Bonekamp D, Deike K, Wiestler B et al (2015) Association of overall survival in patients with newly diagnosed glioblastoma with contrast-enhanced perfusion MRI: comparison of intraindividually matched T1-and T2*-based bolus techniques. J Magn Reson Imaging 42:87–96
Jensen RL, Mumert ML, Gillespie DL, Kinney AY, Schabel MC, Salzman KL (2014) Preoperative dynamic contrast-enhanced MRI correlates with molecular markers of hypoxia and vascularity in specific areas of intratumoral microenvironment and is predictive of patient outcome. Neuro Oncol 16:280–291
Choi YS, Kim DW, Lee SK et al (2015) The added prognostic value of preoperative dynamic contrast-enhanced MRI histogram analysis in patients with glioblastoma: analysis of overall and progression-free survival. AJNR Am J Neuroradiol 36:2235–2241
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neropathol 131:803–820
Weller M, van den Bent M, Hopkins K et al (2014) EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol 15:e395–e403
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Bjornerud A, Sorensen AG, Mouridsen K, Emblem KE (2011) T1- and T*2-dominant extravasation correction in DSC-MRI: part I—theoretical considerations and implications for assessment of tumor hemodynamic properties. J Cereb Blood Flow Metab 31:2041–2053
Haacke EM, Filleti CL, Gattu R et al (2007) New algorithm for quantifying vascular changes in dynamic contrast-enhanced MRI independent of absolute T1 values. Magn Reson Med 58:463–472
Pluim JPW, Maintz JBA, Viergever MA (2003) Mutual-information-based registration of medical images: a survey. IEEE Trans Med Imaging 22:986–1004
Sundar H, Shen D, Biros G, Xu C, Davatzikos C (2007) Robust computation of mutual information using spatially adaptive meshes. Med Image Comput Comput Assist Interv 10:950–958
Goel MK, Khanna P, Kishore J (2010) Understanding survival analysis: Kaplan-Meier estimate. Int J Ayurveda Res 14:274–278
Cox DR (1972) Regression models and life-tables. J R Stat Soc Series B Stat Methodol 34:187–202
Man MZ, Dyson G, Johnson K, Liao B (2004) Evaluating methods for classifying expression data. J Biopharm Stat 14:1065–1084
van Dijken BR, van Laar PJ, Holtman GA, van der Hoorn A (2017) Diagnostic accuracy of magnetic resonance imaging techniques for treatment response evaluation in patients with high-grade glioma, a systematic review and meta-analysis. Eur Radiol 27:4129–4144
Dominietto M, Dobosz M, Bürgi S et al (2017) Quantification of antiangiogenic treatment effects on tissue heterogeneity in glioma tumour xenograft model using a combination of DCE-MRI and 3D-ultramicroscopy. Eur Radiol 27:2894–2902
Dubois LG, Campanati L, Righy C et al (2014) Gliomas and the vascular fragility of the blood brain barrier. Front Cell Neurosci 8:418
Olar A, Wani KM, Alfaro-Munoz KD et al (2015) IDH mutation status and role of WHO grade and mitotic index in overall survival in grade II–III diffuse gliomas. Acta Neuropathol 129:585–596
Hartmann C, Hentschel B, Wick W et al (2010) Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol 120:707–718
Laperriere N, Zuraw L, Cairncross G (2002) Radiotherapy for newly diagnosed malignant glioma in adults: a systematic review. Radiother Oncol 64:259–273
Laws ER, Parney IF, Huang W et al (2003) Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg 99:467–473
Walker MD, Alexander E Jr, Hunt WE et al (1978) Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas: a cooperative clinical trial. J Neurosurg 49:333–343
Lanvin O, Monferran S, Delmas C, Couderc B, Toulas C, Cohen-Jonathan-Moyal E (2013) Radiation-induced mitotic cell death and glioblastoma radioresistance: a new regulating pathway controlled by integrin-linked kinase, hypoxia-inducible factor 1alpha and survivin in U87 cells. Eur J Cancer 49:2884–2891
Siemann DW, Warrington KH, Horsman MR (2000) Targeting tumor blood vessels: an adjuvant strategy for radiation therapy. Radiother Oncol 57:5–12
Park HJ, Griffin RJ, Hui S, Levitt SH, Song CW (2012) Radiation-induced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiat Res 177:311–327
Wilson WR, Hay MP (2011) Targeting hypoxia in cancer therapy. Nat Rev Cancer 11:393
Røe K, Mikalsen LT, van der Kogel AJ et al (2012) Vascular responses to radiotherapy and androgen-deprivation therapy in experimental prostate cancer. Radiat Oncol 7:75
Koukourakis MI, Giatromanolaki A, Sivridis E, Fezoulidis I (2000) Cancer vascularization: implications in radiotherapy? Int J Radiat Oncol Biol Phys 48:545–553
Jacquemier JD, Penault Llorca FM, Bertucci F et al (1998) Angiogenesis as a prognostic marker in breast carcinoma with conventional adjuvant chemotherapy: a multiparametric and immunohistochemical analysis. J Pathol 184:130–135
Cooper RA, Wilks DP, Logue JP et al (1998) High tumor angiogenesis is associated with poorer survival in carcinoma of the cervix treated with radiotherapy. Clin Cancer Res 4:2795–2800
Connolly DT, Heuvelman DM, Nelson R et al (1989) Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest 84:1470–1478
Li X, Zhu Y, Kang H et al (2015) Glioma grading by microvascular permeability parameters derived from dynamic contrast-enhanced MRI and intratumoral susceptibility signal on susceptibility weighted imaging. Cancer Imaging 15:4
Pike MM, Stoops CN, Langford CP, Akella NS, Nabors LB, Gillespie GY (2009) High-resolution longitudinal assessment of flow and permeability in mouse glioma vasculature: sequential small molecule and SPIO dynamic contrast agent MRI. Magn Reson Med 61:615–625
Zhang J, Liu H, Tong H et al (2017) Clinical applications of contrast-enhanced perfusion MRI techniques in gliomas: recent advances and current challenges. Contrast Media Mol Imaging 2017:7064120
Yang L, Krefting I, Gorovets A et al (2012) Nephrogenic systemic fibrosis and class labeling of gadolinium-based contrast agents by the Food and Drug Administration. Radiology 265:248–253
McDonald RJ, McDonald JS, Dai D et al (2017) Comparison of gadolinium concentrations within multiple rat organs after intravenous administration of linear versus macrocyclic gadolinium chelates. Radiology 285:536–545
McGehee BE, Pollock JM, Maldjian JA (2012) Brain perfusion imaging: how does it work and what should I use? J Magn Reson Imaging 36:1257–1272
Sourbron SP, Buckley DL (2011) On the scope and interpretation of the Tofts models for DCE-MRI. Magn Reson Med 66:735–745
Sourbron SP, Buckley DL (2011) Tracer kinetic modelling in MRI: estimating perfusion and capillary permeability. Phys Med Biol 57:R1
Sourbron S, Ingrisch M, Siefert A, Reiser M, Herrmann K (2009) Quantification of cerebral blood flow, cerebral blood volume, and blood-brain-barrier leakage with DCE-MRI. Magn Reson Med 62:205–217
Funding
This study was supported by a grant from the Korea Healthcare Technology R&D Projects, Ministry for Health, Welfare & Family Affairs (HI16C1111); by the Bio & Medical Technology Development Program of the NRF funded by the Korean government, MSIP (NRF-2015M3A9A7029740); by the Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2016M3C7A1914002); by the Creative-Pioneering Researchers Program through Seoul National University (SNU); and by Project Code (IBS-R006-D1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Seung Hong Choi.
Conflict of interest
The authors declare that they have no conflict of interest.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the institutional review board.
Ethical approval
Institutional review board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in Radiology, 2018 Mar; 286(3):981–991.
Methodology
• Retrospective
• Diagnostic or prognostic study
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, H.S., Kwon, S.L., Choi, S.H. et al. Prognostication of anaplastic astrocytoma patients: application of contrast leakage information of dynamic susceptibility contrast-enhanced MRI and dynamic contrast-enhanced MRI. Eur Radiol 30, 2171–2181 (2020). https://doi.org/10.1007/s00330-019-06598-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06598-7