Abstract
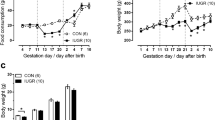
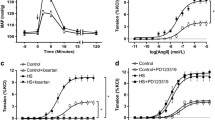
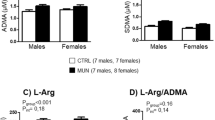
Intrauterine growth restriction (IUGR) affects vascular reactivity in older rats, but at present the causative factors for this change are unknown. Therefore, we investigated downstream events associated with vascular reactivity, specifically, Ca2+-regulated force production and shifts in contractile protein content. The mesenteric artery from male and female 1-year-old Wistar-Kyoto rats was examined using two distinct experimental growth restriction models. Uterine ligation surgery restriction or a sham surgery was conducted at day 18 of pregnancy, whilst a food restriction diet (40% control diet) began on gestational day 15. Extracellular vascular reactivity was studied using intact mesenteric arteries, which were subsequently chemically permeabilized using 50 μM β-escin to examine Ca2+-activated force. Peak contractile responses to a K+-induced depolarization and phenylephrine were significantly elevated due to an increase in maximum Ca2+-activated force in the male surgery restricted group. No changes in contractile forces were reported between female experimental groups. Sections of mesenteric artery were examined using western blotting, revealing IUGR increased the relative abundance of the voltage-gated Ca2+ channel, inositol-1,4,5-trisphosphate receptor and myosin light chain kinase, in both male growth restricted groups, whereas no changes were seen in females. These findings demonstrate for the first time in 1-year-old rats that changes in vascular reactivity due to IUGR are caused by a change in Ca2+-activated force and shifts in important contractile protein content. These changes affect the Wistar-Kyoto rat in a sex-specific and maternal insult-dependent manner.




Similar content being viewed by others
Abbreviations
- ACh:
-
Acetylcholine
- C:
-
Control
- CaV1.2:
-
α1C L-type voltage-gated Ca2+-channel
- EGTA:
-
Ethylene glycol bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid
- FR:
-
Food restricted
- IP3R1:
-
Inositol trisphosphate receptor 1
- IUGR:
-
Intrauterine growth restriction
- MLC:
-
Myosin light chain
- MLCK:
-
Myosin light chain kinase
- MLCP:
-
Myosin light chain phosphatase
- Myh11:
-
Smooth muscle myosin heavy chain
- Mypt1:
-
Myosin light chain phosphatase target subunit 1
- PE:
-
Phenylephrine
- PSS:
-
Physiological saline solution
- SC:
-
Surgical control
- SR:
-
Surgical restricted
- TBST:
-
Tris buffered saline-Tween
- TPM4:
-
Tropomyosin 4
- WKY:
-
Wistar-Kyoto
References
Abou-Saleh H, Pathan AR, Daalis A, Hubrack S, Abou-Jassoum H, Al-Naeimi H, Rusch NJ, Machaca K (2013) Inositol 1, 4, 5-trisphosphate (IP3) receptor up-regulation in hypertension is associated with sensitization of Ca2+ release and vascular smooth muscle contractility. J Biol Chem 288:32941–32951
Anderson CM, Lopez F, Zimmer A, Benoit JN (2006) Placental insufficiency leads to developmental hypertension and mesenteric artery dysfunction in two generations of Sprague-Dawley rat offspring. Biol Reprod 74:538–544
Barker D, Osmond C, Golding J, Kuh D, Wadsworth M (1989) Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 298:564–567
Bergmann R, Bergmann K, Dudenhausen J (2008) Undernutrition and growth restriction in pregnancy. In: The window of opportunity: pre-pregnancy to 24 months of age. Karger Publishers, Basel, pp 103–121
Bourque SL, Gragasin FS, Quon AL, Mansour Y, Morton JS, Davidge ST (2013) Prenatal hypoxia causes long-term alterations in vascular endothelin-1 function in aged male, but not female, offspring. Hypertension 62:753–758
Brawley L, Itoh S, Torrens C, Barker A, Bertram C, Poston L, Hanson M (2003) Dietary protein restriction in pregnancy induces hypertension and vascular defects in rat male offspring. Pediatr Res 54:83
Bubb KJ, Cock ML, Black MJ, Dodic M, Boon WM, Parkington HC, Harding R, Tare M (2007) Intrauterine growth restriction delays cardiomyocyte maturation and alters coronary artery function in the fetal sheep. J Physiol 578:871–881
Camm EJ, Hansell JA, Kane AD, Herrera EA, Lewis C, Wong S, Morrell NW, Giussani DA (2010) Partial contributions of developmental hypoxia and undernutrition to prenatal alterations in somatic growth and cardiovascular structure and function. Am J Obstet Gynecol 203:495–e24
Christie MJ, Romano T, Murphy RM, Posterino GS (2018) The effect of intrauterine growth restriction on Ca2+-activated force and contractile protein expression in the mesenteric artery of adult (6-month-old) male and female Wistar-Kyoto rats. Physiol Rep 6:e13954
Gluckman PD, Hanson MA, Cooper C, Thornburg KL (2008) Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359:61–73
Han Y-J, Hu W-Y, Chernaya O, Antic N, Gu L, Gupta M, Piano M, De Lanerolle P (2006) Increased myosin light chain kinase expression in hypertension: regulation by serum response factor via an insertion mutation in the promoter. Mol Biol Cell 17:4039–4050
Harvey TJ, Murphy RM, Morrison JL, Posterino GS (2015) Maternal nutrient restriction alters Ca2+ handling properties and contractile function of isolated left ventricle bundles in male but not female juvenile rats. PLoS One 10:e0138388
Hemmings DG, Williams SJ, Davidge ST (2005) Increased myogenic tone in 7-month-old adult male but not female offspring from rat dams exposed to hypoxia during pregnancy. Am J Physiol Heart Circ Physiol 289:H674–H682
House SJ, Potier M, Bisaillon J, Singer HA, Trebak M (2008) The non-excitable smooth muscle: calcium signaling and phenotypic switching during vascular disease. Pflugers Arch 456:769–785
Kudryavtseva O, Herum KM, Dam VS, Straarup MS, Kamaev D, Briggs Boedtkjer DM, Matchkov VV, Aalkjær C (2014) Downregulation of L-type Ca2+ channel in rat mesenteric arteries leads to loss of smooth muscle contractile phenotype and inward hypertrophic remodeling. Am J Physiol Heart Circ Physiol 306:H1287–H1301
Mazzuca MQ, Wlodek ME, Dragomir NM, Parkington HC, Tare M (2010) Uteroplacental insufficiency programs regional vascular dysfunction and alters arterial stiffness in female offspring. J Physiol 588:1997–2010
Murphy RM, Lamb GD (2013) Important considerations for protein analyses using antibody based techniques: down-sizing Western blotting up-sizes outcomes. J Physiol 591:5823–5831
O'Dowd R, Kent JC, Moseley JM, Wlodek ME (2008) Effects of uteroplacental insufficiency and reducing litter size on maternal mammary function and postnatal offspring growth. Am J Physiol Regul Integr Comp Physiol 294:R539–R548
Owens GK, Kumar MS, Wamhoff BR (2004) Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84:767–801
Ozaki T, Nishina H, Hanson M, Poston L (2001) Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. J Physiol 530:141–152
Ratz PH, Berg KM, Urban NH, Miner AS (2005) Regulation of smooth muscle calcium sensitivity: KCl as a calcium-sensitizing stimulus. Am J Physiol Cell Physiol 288:C769–C783
Resnik R (2013) Fetal growth restriction: evaluation and management. Obstet Gynecol 99:490–496
Rodríguez-Rodríguez P, Ramiro-Cortijo D, Reyes-Hernández CG, de Pablo ALL, González MC, Arribas SM (2018) Implication of oxidative stress in fetal programming of cardiovascular disease. Front Physiol 9:602
Sathishkumar K, Elkins R, Yallampalli U, Yallampalli C (2009) Protein restriction during pregnancy induces hypertension and impairs endothelium-dependent vascular function in adult female offspring. J Vasc Res 46:229–239
Tare M, Parkington HC, Bubb KJ, Wlodek ME (2012) Uteroplacental insufficiency and lactational environment separately influence arterial stiffness and vascular function in adult male rats. Hypertension 60:378–386
Thompson LP, Al-Hasan Y (2013) Impact of oxidative stress in fetal programming. J Pregnancy 2012:582748
Touyz RM, Alves-Lopes R, Rios FJ, Camargo LL, Anagnostopoulou A, Arner A, Montezano AC (2018) Vascular smooth muscle contraction in hypertension. Cardiovasc Res 114:529–539
Williams SJ, Campbell ME, McMillen IC, Davidge ST (2005) Differential effects of maternal hypoxia or nutrient restriction on carotid and femoral vascular function in neonatal rats. Am J Physiol Regul Integr Comp Physiol 288:R360–R367
Williams SJ, Hemmings DG, Mitchell JM, McMillen IC, Davidge ST (2005) Effects of maternal hypoxia or nutrient restriction during pregnancy on endothelial function in adult male rat offspring. J Physiol 565:125–135
Wlodek ME, Mibus A, Tan A, Siebel AL, Owens JA, Moritz KM (2007) Normal lactational environment restores nephron endowment and prevents hypertension after placental restriction in the rat. J Am Soc Nephrol 18:1688–1696
Wlodek ME, Westcott KT, O’Dowd R, Serruto A, Wassef L, Moritz KM, Moseley JM (2005) Uteroplacental restriction in the rat impairs fetal growth in association with alterations in placental growth factors including PTHrP. Am J Physiol Regul Integr Comp Physiol 288:R1620–R1627
Acknowledgments
The authors would like to thank Maria Cellini, Heidy Flores, and Aida Youssef for technical assistance, and Simon Watson for statistical analysis design.
Funding
All funding provided by La Trobe University; WBS 4.0062.10.35.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• IUGR increased maximum Ca2+-activated force in male resistance arteries
• Concurrent increase in contractile protein expression of male IUGR arteries
• No contractile or biochemical changes in female IUGR mesenteric arteries
• Sex-specific and maternal insult-dependent effect of IUGR on resistance arteries
Electronic supplementary material
ESM 1
(DOCX 405 kb).
Rights and permissions
About this article
Cite this article
Christie, M.J., Romano, T., Murphy, R.M. et al. Effects of intrauterine growth restriction on Ca2+-activated force and contractile protein expression in the mesenteric artery of 1-year-old Wistar-Kyoto rats. J Physiol Biochem 76, 111–121 (2020). https://doi.org/10.1007/s13105-020-00724-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-020-00724-6