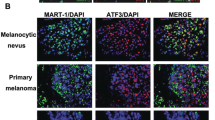
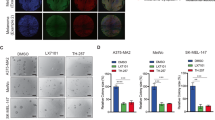
Abstract
The BRAFV600E mutation occurs in more than 50% of cutaneous melanomas, and results in the constitutive activation of the mitogen-activated protein kinases (MAPK) pathway. MAP kinase-interacting serine/threonine-protein kinase 1 and 2 (MNK1/2) are downstream effectors of the activated MAPK pathway, and important molecular targets in invasive and metastatic cancer. Despite the well-known role of MNK1 in regulating mRNA translation, little is known concerning the impact of its aberrant activation on gene transcription. Here, we show that changes in the activity, or abundance, of MNK1 result in changes in the expression of pro-oncogenic and pro-invasive genes. Among the MNK1-upregulated genes, we identify Angiopoietin-like 4 (ANGPTL4), which in turn promotes an invasive phenotype via its ability to induce the expression of matrix metalloproteinases (MMPs). Using a pharmacologic inhibitor of MNK1/2, SEL201, we demonstrate that BRAFV600E-mutated cutaneous melanoma cells are reliant on MNK1/2 for invasion and lung metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Larkin J, Ascierto PA, Dreno B, Atkinson V, Liszkay G, Maio M, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867–76.
Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–9.
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23.
Larkin J, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:1270–1.
Domingues B, Lopes JM, Soares P, Populo H. Melanoma treatment in review. Immunotargets Ther. 2018;7:35–49.
Geller AC, Annas GD. Epidemiology of melanoma and nonmelanoma skin cancer. Semin Oncol Nurs. 2003;19:2–11.
Cancer Genome Atlas N. Genomic classification of cutaneous melanoma. Cell. 2015;161:1681–96.
Zhan Y, Guo J, Yang W, Goncalves C, Rzymski T, Dreas A, et al. MNK1/2 inhibition limits oncogenicity and metastasis of KIT-mutant melanoma. J Clin Investig. 2017;127:4179–92.
Robichaud N, Del Rincon SV, Huor B, Alain T, Petruccelli LA, Hearnden J, et al. Phosphorylation of eIF4E promotes EMT and metastasis via translational control of SNAIL and MMP-3. Oncogene. 2015;34:2032–42.
Ramalingam S, Gediya L, Kwegyir-Afful AK, Ramamurthy VP, Purushottamachar P, Mbatia H, et al. First MNKs degrading agents block phosphorylation of eIF4E, induce apoptosis, inhibit cell growth, migration and invasion in triple negative and Her2-overexpressing breast cancer cell lines. Oncotarget. 2014;5:530–43.
Guo Z, Peng G, Li E, Xi S, Zhang Y, Li Y, et al. MAP kinase-interacting serine/threonine kinase 2 promotes proliferation, metastasis, and predicts poor prognosis in non-small cell lung cancer. Sci Rep. 2017;7:10612.
Guo Q, Li VZ, Nichol JN, Huang F, Yang W, Preston SEJ, et al. MNK1/NODAL signaling promotes invasive progression of breast ductal carcinoma in situ. Cancer Res. 2019;79:1646–57.
Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34.
Joshi S, Platanias LC. Mnk kinase pathway: cellular functions and biological outcomes. World J Biol Chem. 2014;5:321–33.
Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE Jr., et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41:544–52.
Liu L, Zhuang X, Jiang M, Guan F, Fu Q, Lin J. ANGPTL4 mediates the protective role of PPARgamma activators in the pathogenesis of preeclampsia. Cell Death Dis. 2017;8:e3054.
Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278:16–27.
Hu K, Babapoor-Farrokhran S, Rodrigues M, Deshpande M, Puchner B, Kashiwabuchi F, et al. Hypoxia-inducible factor 1 upregulation of both VEGF and ANGPTL4 is required to promote the angiogenic phenotype in uveal melanoma. Oncotarget. 2016;7:7816–28.
Wang JH, Huang WS, Hu CR, Guan XX, Zhou HB, Chen LB. Relationship between RGS5 expression and differentiation and angiogenesis of gastric carcinoma. World J Gastroenterol. 2010;16:5642–6.
Redondo M, Tellez T, Roldan MJ. The role of clusterin (CLU) in malignant transformation and drug resistance in breast carcinomas. Adv Cancer Res. 2009;105:21–43.
Baron V, Adamson ED, Calogero A, Ragona G, Mercola D. The transcription factor Egr1 is a direct regulator of multiple tumor suppressors including TGFbeta1, PTEN, p53, and fibronectin. Cancer Gene Ther. 2006;13:115–24.
Liao YH, Chiang KH, Shieh JM, Huang CR, Shen CJ, Huang WC, et al. Epidermal growth factor-induced ANGPTL4 enhances anoikis resistance and tumour metastasis in head and neck squamous cell carcinoma. Oncogene. 2017;36:2228–42.
Georgiadi A, Lichtenstein L, Degenhardt T, Boekschoten MV, van Bilsen M, Desvergne B, et al. Induction of cardiac Angptl4 by dietary fatty acids is mediated by peroxisome proliferator-activated receptor beta/delta and protects against fatty acid-induced oxidative stress. Circ Res. 2010;106:1712–21.
Fang L, Zhang M, Li Y, Liu Y, Cui Q, Wang N. PPARgene: a database of experimentally verified and computationally predicted PPAR target genes. PPAR Res. 2016;2016:6042162.
Naruhn S, Toth PM, Adhikary T, Kaddatz K, Pape V, Dorr S, et al. High-affinity peroxisome proliferator-activated receptor beta/delta-specific ligands with pure antagonistic or inverse agonistic properties. Mol Pharm. 2011;80:828–38.
Djeu JY, Wei S. Clusterin and chemoresistance. Adv Cancer Res. 2009;105:77–92.
Kimura R, Ishikawa C, Rokkaku T, Janknecht R, Mori N. Phosphorylated c-Jun and Fra-1 induce matrix metalloproteinase-1 and thereby regulate invasion activity of 143B osteosarcoma cells. Biochim Biophys Acta. 2011;1813:1543–53.
Korneeva NL, Soung YH, Kim HI, Giordano A, Rhoads RE, Gram H, et al. Mnk mediates integrin alpha6beta4-dependent eIF4E phosphorylation and translation of VEGF mRNA. Mol Cancer Res. 2010;8:1571–8.
Ascierto PA, Kirkwood JM, Grob JJ, Simeone E, Grimaldi AM, Maio M, et al. The role of BRAF V600 mutation in melanoma. J Transl Med. 2012;10:85.
O’Loghlen A, Gonzalez VM, Jurado T, Salinas M, Martin ME. Characterization of the activity of human MAP kinase-interacting kinase Mnk1b. Biochim Biophys Acta. 2007;1773:1416–27.
La Paglia L, Listi A, Caruso S, Amodeo V, Passiglia F, Bazan V, et al. Potential role of ANGPTL4 in the cross talk between metabolism and cancer through PPAR signaling pathway. PPAR Res. 2017;2017:8187235.
Beyaz S, Yilmaz OH. Molecular pathways: dietary regulation of stemness and tumor initiation by the PPAR-delta pathway. Clin Cancer Res. 2016;22:5636–41.
Vella V, Nicolosi ML, Giuliano S, Bellomo M, Belfiore A, Malaguarnera R. PPAR-gamma agonists as antineoplastic agents in cancers with dysregulated IGF axis. Front Endocrinol. 2017;8:31.
Pettersson F, Del Rincon SV, Emond A, Huor B, Ngan E, Ng J, et al. Genetic and pharmacologic inhibition of eIF4E reduces breast cancer cell migration, invasion, and metastasis. Cancer Res. 2015;75:1102–12.
Acknowledgements
This research is funded by the Canadian Institutes for Health Research (grants MOP-142281 PJT-156269 to WHM and SVDR, and grant PJT-162260 to SVDR) and the Canadian Cancer Society (grant 703811 WHM and SVDR). The research was further supported by the Rossy Cancer Network. Development of MNK1/2 inhibitors by Selvita S.A. has been co-financed by the National Centre for Research and Development, INNOTECH Program (INNOTECH-K1/HI1/I6/157438/NCBR/12). WY was endowed by MICRTP graduate studentships. QG was financed by a Cole Foundation Ph.D. fellowship, McGill Integrated Cancer Research Training Program (MICRTP) graduate studentship and a McGill Faculty of Medicine graduate studentship. FH was sponsored by MICRTP graduate studentships. We thank Shalom Chaim Spira for editing this manuscript.
Author information
Authors and Affiliations
Contributions
WY, EK, QG, WHM, and SVDR designed the research. WY, EK, QG, SAP, FH, CG, DP, and YZ performed the experiments and analyzed the data. AE analyzed the RNA-Seq data. WY, EK, QG, FH, JNN, MSD, WHM, and SVDR wrote and edited the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, W., Khoury, E., Guo, Q. et al. MNK1 signaling induces an ANGPTL4-mediated gene signature to drive melanoma progression. Oncogene 39, 3650–3665 (2020). https://doi.org/10.1038/s41388-020-1240-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-020-1240-5
This article is cited by
-
The role of eIF4F-driven mRNA translation in regulating the tumour microenvironment
Nature Reviews Cancer (2023)
-
Evolution of Biomarkers and Treatment Outcomes of Immunotherapy in Lung Cancer
Current Tissue Microenvironment Reports (2023)
-
MNK1 and MNK2 enforce expression of E2F1, FOXM1, and WEE1 to drive soft tissue sarcoma
Oncogene (2021)
-
Identification and validation of a six-gene signature associated with glycolysis to predict the prognosis of patients with cervical cancer
BMC Cancer (2020)