Abstract
Background
The diagnosis of IBD and evaluation of treatment require endoscopy, which is difficult in children. This study evaluated the use of TFF3 as a biomarker.
Methods
Permeability of the intestinal mucosa and serum TFF3 were assayed and colon tissue was harvested 7 days after inducing IBD in mice with TNBSA. TFF3 was monitored in 51 pediatric IBD patients stratified by active disease or remission and in 20 healthy children. Mucosal healing was assessed by the Simple Endoscopic Score for Crohn Disease and Baron scores in CD and UC patients.
Results
Histological evaluation revealed transmural inflammation of the colon in IBD model mice. Permeability of the intestinal mucosa and serum TFF3 were both higher in TNBSA-treated than in control mice (P < 0.05). TFF3 was higher in children with active IBD than in those in remission and in healthy children (P < 0.05). TFF3 was positively correlated with the SES-CD score (P < 0.05) but not with either the pediatric CDAI score or the serum CRP. The sensitivity of serum TFF3 for monitoring CD activity was 100% and the specificity was 76.2%.
Conclusions
TFF3 level increased with CD activity, which is of significance for diagnosis and for evaluation of mucosal healing. TFF3 could also be a marker in pediatric UC, as TFF3 positively correlated with UCAI.
Impact
-
The diagnosis and evaluation of IBD is difficult; endoscopy provides objective assessment; TFF3 can be a useful marker instead of endoscopy.
-
TFF3 was increased in active CD of children; TFF3 can be used as a clinical marker of pediatric CD; TFF3 can diagnose and evaluate mucosal healing of CD.
-
Pediatrician should pay attention to clinical marker; TFF3 level may be a key evaluation of mucosal healing of CD; the value of diagnosis of TFF3 in CD is important.
Similar content being viewed by others
Introduction
The incidence of inflammatory bowel diseases (IBDs), including both Crohn’s disease (CD) and ulcerative colitis (UC), is relatively high in North America and Europe, and its morbidity in China is increasing.1,2,3,4,5,6 IBD is of pediatric interest because it is an important reason for failure to thrive and decline in the quality of life.7,8,9 The etiology and pathogenesis of IBD are not clearly understood but depend on the interaction of genetic, environmental, and immunological factors leading to intestinal epithelial barrier dysfunction.10,11,12 The diagnosis of IBD is challenging and requires laboratory testing, imaging, endoscopy, and pathological evaluation in addition to evaluation of clinical manifestations.13,14 The goal of treatment is mucosal healing rather than achieving and maintaining clinical remission.15,16 Patients without mucosal healing have an increased probability of recurrence, and about 30% of patients ultimately require surgery.17,18 Endoscopy provides an objective assessment of IBD status, but it is invasive, with heavy economic and psychological burden if it needs to be repeated frequently. It is also not free of adverse events. The risk of anesthesia is higher in children than in adults. The intestinal wall of children is thin and fragile and the lumen is narrow, both of which increase the risk of severe complications. Novel reliable and noninvasive markers are urgently needed. Recently, fecal calprotectin (FC) has been used to screen IBD.19 Calprotectin belongs to the S100 family and is derived predominantly from neutrophils. Thus, the presence of elevated FC is an indicator of mucosal neutrophil infiltration.20 In addition, various factors may affect FC results, including age, medication and day-to-day variation.21 Finding a specific biomarker expressed in the diseased intestine may have more diagnostic value. Trefoil factor peptide 3 (TFF3) is a peptide specifically secreted by intestinal goblet cells. It promotes cell migration and proliferation, repair of mucosal epithelium, and angiogenesis, which act to maintain the intestinal epithelial barrier and normal intestinal function.22 Both in vitro and animal studies indicate that TFF3 can maintain the integrity of the intestinal epithelium and regulate mucosal inflammation.23,24,25 TFF3 may have therapeutic potential for IBD, and serum TFF3 levels have been investigated as a biomarker of mucosal healing in adults with UC.26,27 As studies in children are lacking, investigations of TFF3 as a pediatric biomarker are warranted.
Materials and methods
Animal model of IBD
The increase in serum TFF3 following damage to the intestinal mucosal barrier was investigated in a TNBSA-induced mouse IBD model, which is widely used to replicate impairment of the intestinal mucosal barrier. Intestinal permeability was determined by measuring the fluorescence of isothiocyanate-conjugated dextran (FD-4) following gavage into the lumen of the intestine. Serum TFF3 concentration was also assayed.
Procedures for care and handling of animals used in this study were approved by the ethics committee of China Medical University. Twenty female BALB/C mice (SCXK shen 2015-0001) between 5 and 8 weeks of age were used. The mice were randomly assigned in equal numbers to an IBD group given a single intracolonic dose of 0.2 mL of 50% ethanol (vol/vol) containing 25 mg/mL TNBSA (Sigma-Aldrich) or a control group given the same volume of normal saline instead of TNBSA in ethanol.28 On day 7 after induction of colitis, 0.5 mL FD-4 (molecular weight 4000, 40 mg/mL) was gavaged into the lumen of the intestine using a gavage needle. The mice were killed after 30 min, a 100 µL blood sample was collected, mixed with 1.9 mL 50 mol/L Tris solution and centrifuged at 4000 × g for 10 min at 4 °C. The FD-4 that entered the blood from the intestine was measured spectrofluorometrically with an excitation wavelength of 480 nm and emission wavelength of 520 nm using a microplate fluorescence reader (FLX-800; BioTek Instruments, Winooski, VT, USA). FD-4 permeability was reported as µg/mL. Tissue samples were collected for assays and serum TFF3 concentration was measured with a commercial sandwich enzyme-linked immunosorbent assay (Mouse TTF3 ELISA, Boster Biological) kit, following the manufacturer’s instructions.
Pediatric IBD patients
The study cohort included 51 children, 28 boys and 23 girls from 5 to 17 years of age with IBD and treated at Shengjing Hospital of China Medical University between March 2017 and September 2018. Twenty healthy children were selected during the study period as the normal control group. All children were diagnosed at our hospital and all met the 2010 diagnostic criteria established by the Digestive Science Group, Pediatrics Branch, of the Chinese Medical Association.29 The growth and clinical characteristics, laboratory investigations, endoscopic examination, histological features, therapy and outcome were reviewed.
Blood samples were collected the morning after admission for assay of serum TFF3 using a commercial sandwich ELISA kit (Human TTF3 UCSN Life Science İnc. USA). Clinical disease activity of UC was described with the Pediatric Ulcerative Colitis Activity Index (PUCAI).30 The index is scored from 0 to 85, with clinical remission as <10, mild activity as 10–34, moderate activity as 35–64, and severe activity as >65. The clinical disease activity of CD was described by the pediatric Crohn’s Disease Activity Index (PCDAI); 0–10 indicated inactive disease, 10–30 indicated mild disease, and >30 indicated moderate-to-severe disease.31 In patients with luminal Crohn’s disease, activity was evaluated endoscopically using the simple endoscopic score for Crohn’s Disease (SES-CD); 0 or 1 indicated mucosal healing.32 Mucosal changes associated with UC were evaluated by Baron’s scoring system.33 Grade 0 or 1 changes indicated mucosal healing.
Ethical approval and consent to participate
The study protocol was approved by the Ethics Committee of Shengjing Hospital of China Medical University (approval no. 2015PS281K Shenyang China). Informed consent was obtained from each patient following the institutional guidelines.
Statistical analysis
Statistical analysis was performed with SPSS 22.0 (IBM Corp., Armonk, NY, USA). Measurement data were reported as means ± standard deviation. Differences in patient group means were compared with the Mann−Whitney U test. One-way analysis of variance and independent t tests were used to compare differences between the mouse groups. Correlations were tested for significance by calculating the Spearman’s coefficient. Receiver operating characteristic (ROC) curves were derived from the original data and the maximum value of the Jordan index (sensitivity + specificity – 1) was used as the diagnostic cutoff value. P values < 0.05 was considered statistically significant.
Results
Animal model of IBD
Mice in the TNBSA group had diarrhea and severe weight loss. Histological manifestations included transmural ulceration with complete loss of epithelium and extensive lymphocyte, neutrophil, monocyte, and eosinophil infiltration of the mucosa (Fig. 1).
Serum TFF3 was significantly higher in the TNBSA-treated than the control mice (P < 0.05). Serum FD-4 fluorescence was significantly higher in the TNBSA-treated than the control mice (P < 0.01, Table 1).
IBD patients
In this cohort study, 33 of the 51 IBD patients (64.7%) had CD; 18 (35.3%) had UC; 24 (47%) had active IBD, and 27 (53%) were in remission. The PCDAI scores indicated that 15 CD patients had active disease and 18 were in the remission stage. The PUCAI scores indicated active disease and remission in nine patients each. There were no significant differences in age or sex in the healthy control and IBD groups (Table 2). There were no significant differences in serum TFF3 in CD and UC patients. Serum TFF3 was significantly higher in patients with active IBD than in both the controls and the IBD patients in remission. Serum CRP was significantly higher in patients with active CD than in both the controls and remission group (P < 0.01, Table 3).
Correlation of serum TFF3 and CRP with PCDAI and SES-CD scores in CD patients
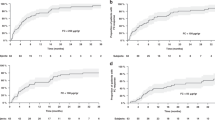
Serum TFF3 was positively correlated with the SES-CD score (r = 0.659, P = 0.007), but not with PCDAI (r = 0.472, P = 0.076). CRP was not significantly correlated with the SES-CD score (r = 0.412, P = 0.082). ROC curve analysis yielded an area under the ROC curve of 0.926 for serum TFF3. The sensitivity of TFF3 to detect active IBD in this pediatric sample was 100% and the specificity was 76.2% (Fig. 2 and Table 4). The TFF3 concentration reflected the extent of mucosal healing in the CD patients.
Correlation of serum TFF3 and CRP with PUCAI and Baron’s scores in UC patients
Serum TFF3 was positively correlated with PUCAI (r = 0.994, P = 0.000) but not with serum CRP (r = 0.419, P = 0.301), or Baron’s score (r = 0.478, P = 0.230).
Discussion
In this study, serum TFF3 was assayed in both IBD model mice and pediatric IBD patients. Serum TFF3 was significantly higher in model mice with high epithelial permeability. Serum TFF3 levels were also elevated in patients in the active disease stage and in those who had not achieved mucosal healing. We propose TFF3 as a new biomarker in pediatric IBD.
In this study, the colon mucosa was destroyed in the IBD model mice. The significant increase of serum FD-4 fluorescence confirmed that the intestinal mucosa had been damaged and the TFF3 concentration in peripheral blood significantly increased in parallel with damage to the intestinal barrier. TFF3 is secreted by intestinal goblet cells and is normally present in very small amounts in the blood. Can large amounts of TFF3 come from the damaged intestinal mucosal? We believe that in the IBD model mice, the goblet cells secreted large amounts of TFF3 that entered the blood through the damaged intestinal mucosa. That can be confirmed by following the movement of tracer-labeled TFF3 from damaged intestinal segments into the peripheral blood, which is planned.
An increase in TFF3 in the peripheral blood of pediatric IBD patients that exceeds a threshold level can be considered as a marker of disease activity or recurrence. This hypothesis and potential clinical application of serum TFF3 was tested in a cohort of pediatric IBD patients. Serum TFF3 was significantly higher in patients with active CD and UC disease than those in the remission stage and in the healthy control group. Serum TFF3 was positively correlated with the SES-CD score. Its sensitivity for monitoring CD activity was 100% and its specificity was 76.2%. The predicted cutoff value of serum TFF3 was 6.01 ng/mL. Serum TFF3 level may thus reflect the extent of mucosal healing or damage and serve as a biomarker of mucosal healing in children with CD. A previous study in adults reported a correlation of serum TFF3 and Baron’s score in UC patients, but that was not observed in this group of pediatric patients.24 Possible reasons for the difference are the small number of included UC patients in this study and differences unknown between adult and pediatric patients.
Performing only one serum TFF3 assay was a study limitation. Serial measurements would have allowed monitoring changes that occurred during the progression of disease and possibly determining whether patients with low serum TFF3 at onset or remission had a better prognosis than those with a high initial TFF3 concentration. Additional clinical studies in larger groups of patients are desirable to evaluate the significance of TFF3 as a biomarker in PIBD.
Conclusion
Serum TFF3 level significantly increased with CD activity, which is of significance for diagnosis and evaluation of mucosal healing. TFF3 could also be a marker in pediatric UC with TFF3 positively correlated with UCAI.
References
Ng, S. C. et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390, 2769–2778 (2018).
Guan, D. X. et al. Single center retrospective study of 184 children with inflammatory bowel disease seen from 2000–2014. Zhonghua Er Ke Za Zhi. 55, 493–498 (2017).
Teng, X., Xu, L. F., Sun, M. & Guo, j Phenotypic characteristics and clinical manifestations of inflammatory bowel disease in infants and children under 2 years of age in Liaoning Province, China: five of six infants with IL-10R mutations[J]. Paediatr. Int. Child Health 39, 59–64 (2019).
Ebrahimi, S. M. S. et al. Neonatal presentation of unremitting inflammatory bowel disease. Iran. J. Med. Sci. 43, 328–331 (2018).
Dinwiddie, D. L. et al. Molecular diagnosis of infantile onset inflammatory bowel disease by exome sequencing. Genomics 102, 442–447 (2013).
Al-Qabandi, W. A. et al. Inflammatory bowel disease in children, an evolving problem in Kuwait. Saudi J. Gastroenterol. 17, 323–327 (2011).
Fang, Y. H., Luo, Y. Y., Yu, J. D., Lou, J. G. & Chen, J. Phenotypic and genotypic characterization of inflammatory bowel disease in children under six years of age in China. World J. Gastroenterol. 24, 1035–1045 (2018).
Peng, K. et al. Blood transplantation corrects very early-onset inflammatory bowel disease in Chinese patients with IL10RA-associated immune deficiency. Inflamm. Bowel Dis. 24, 1416–1427 (2018).
Ye, Z. et al. Phenotype and management of infantile-onset inflammatory bowel disease: experience from a tertiary care center in China. Inflamm. Bowel Dis. 23, 2154–2164 (2017).
Sheikh, S., Uno, J., Matsuoka, K. & Plevy, S. Abnormal mucosal immune response to altered bacterial flora following restorative proctocolectomy in patients with ulcerative colitis: serologic measures, immunogenetics, and clinical correlations. Clin. Immunol. 127, 270–279 (2008).
Aschard, H. et al. Genetic effects on the commensal microbiota in inflammatory bowel disease patients. PLoS Genet. 15, e1008018 (2019).
Pei, L. Y. et al. Role of colonic microbiota in the pathogenesis of ulcerative colitis. BMC Gastroenterol. 19, 10 (2019).
Shen, B. The evaluation of postoperative patients with ulcerative colitis. Gastrointest. Endosc. Clin. N. Am. 26, 669–677 (2016).
Ylisaukko-Oja, T. et al. High treatment persistence rate and significant endoscopic healing among real-life patients treated with vedolizumab—a Finnish Nationwide Inflammatory Bowel Disease Cohort Study (FINVEDO). Scand. J. Gastroenterol. 53, 158–167 (2018).
Froslie, K. F., Jahnsen, J., Moum, B. A. & Vatn, M. H., IBSEN Group. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort[J]. Gastroenterology 133, 412–422 (2007).
Rutgeerts, P., Vermeire, S. & Van Assche, G. Mucosal healing in inflammatory bowel disease: impossible ideal or therapeutic target?. Gut 56, 453–455 (2007).
Wu, S. N., Wu, J. & Hang, Y. Clinical utility of capsule endoscopy in 145 pediatric patients[J]. Shanghai Med. J. 5, 275–279 (2017).
Turner, D. et al. Appraisal of the pediatric ulcerative colitis activity index (PUCAI). Inflamm. Bowel Dis. 15, 1218–1223 (2010).
van Rheenen, P. F., de Vijver, E. V. & Fidler, V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease:diagnostic meta-analysis. BMJ 341, c3369 (2010).
Smith, L. A. & Gaya, D. R. Utility of faecal calprotectin analysis in adult inflammatory bowel disease. World J. Gastroenterol. 18, 6782–6789 (2012).
Ayling, R. M. & Kok, K. Fecal calprotectin. Adv. Clin. Chem. 87, 161–190 (2018).
Thim, L. Trefoil peptides: from structure to function. Cell. Mol. Life Sci. 53, 888–903 (1997).
Renes, I. B. et al. Distict epithelial responses in experimental colitis: implications for ion uptake and mucosal protection. Am. J. Physiol. Gastrointest. Liver Physiol. 283, G169–G179 (2002).
Chen, L. P., Zhang, B. H., Li, Y. & Chen, Z. The effect and significance of intestinal trefoil factor IL-8 and MDA for neonatal rat model for hypoxia-induced intestinal injury. Chin. J. Perinat. Med. 36, 306–309 (2003).
Li, J. et al. Protective effects of recombinant intestinal trefoil factor against intestinal injuries induced by endotoxin in young rats. Chin. J. Contemp. Pediatrics 8, 425–428 (2006).
Teng, X., Xu, L. F., Zhou, P., Sun, H. W. & Sun, M. Effects of trefoil peptide 3 on expression of TNF-α, TLR4,and NF-κB in trinitrobenzene sulphonic acid induced colitis mice. Inflammation 32, 120–129 (2009).
Srivastava, S. et al. Serum human trefoil factor 3 is a biomarker for mucosal healing in ulcerative colitis patients with minimal disease activity. J. Crohns Colitis 9, 575–579 (2015).
Stallmach, A. et al. An interleukin 12 p40-IgG2b fusion protein abrogates T cell mediated inflammation: anti-inflammatory activity in Cohn’s disease and experimental colitis in vivo. Gut 53, 339–345 (2004).
Chen, J. et al. Consensus on diagnostic norms for pediatric inflammatory bowel disease. Chin. J. Pract. Pediatrics 25, 263–265 (2010).
Turner, D. et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology 133, 423–432 (2007).
Hyams, J. S. et al. Development and validation of a pediatric Crohn’s disease activity index. J. Pediatr. Gastroenterol. Nutr. 12, 439–447 (1991).
Daperno, M. et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest. Endosc. 60, 505–512 (2004).
Baron, J. H., Connell, A. M. & Lennard-Jones, J. Variation between observers in describing mucosal appearances in proctocolitis. Br. Med. J. 1, 89–92 (1964).
Acknowledgements
This work was supported by the National Natural Science Foundation of China Youth Science Foundation Project (no. 81400585) and Natural Science Foundation of Liaoning Provence, China (grant no. 2019-MS-372).
Author information
Authors and Affiliations
Contributions
X.T. had the primary responsibility for protocol development, outcome assessment, preliminary data analysis and drafting the manuscript. L.L., Y.Y. and L.Y. participated in the development of the protocol, animal experiments, collecting medical records of patient screening and analytical framework for the study and contributed to the writing of the manuscript. L.X., M.S. and J.W. supervised the design and execution of the study and revised the article critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Teng, X., Yang, Y., Liu, L. et al. Evaluation of inflammatory bowel disease activity in children using serum trefoil factor peptide. Pediatr Res 88, 792–795 (2020). https://doi.org/10.1038/s41390-020-0812-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0812-y