Abstract
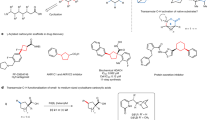
Site-selective functionalization of C–H bonds will ultimately afford chemists transformative tools for editing and constructing complex molecular architectures. Towards this goal, it is essential to develop strategies to activate C–H bonds that are distal from a functional group. In this context, distinguishing remote C–H bonds on adjacent carbon atoms is an extraordinary challenge due to the lack of electronic or steric bias between the two positions. Herein, we report the design of a catalytic system leveraging a remote directing template and a transient norbornene mediator to selectively activate a previously inaccessible remote C–H bond that is one bond further away. The generality of this approach has been demonstrated with a range of heterocycles, including a complex anti-leukaemia agent and hydrocinnamic acid substrates.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information. Metrical parameters for the structure of 2ah (see Supplementary Information) are available free of charge from the Cambridge Crystallographic Data Centre (https://www.ccdc.cam.ac.uk/) under reference number CCDC 1890836.
References
Daugulis, O., Do, H.-Q. & Shabashov, D. Palladium- and copper-catalyzed arylation of carbon–hydrogen bonds. Acc. Chem. Res. 42, 1074–1086 (2009).
Lyons, T. W. & Sanford, M. S. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem. Rev. 110, 1147–1169 (2010).
Abrams, D. J., Provencher, P. A. & Sorensen, E. J. Recent applications of C–H functionalization in complex natural product synthesis. Chem. Soc. Rev. 47, 8925–8967 (2018).
Li, J., Sarkar, S. D. & Ackermann, L. meta- and para-selective C–H functionalization by C–H activation. Top. Organomet. Chem. 55, 217–257 (2016).
Breslow, R. Biomimetic control of chemical selectivity. Acc. Chem. Res. 13, 170–177 (1980).
Das, S., Incarvito, C. D., Crabtree, R. H. & Brudvig, G. W. Molecular recognition in the selective oxygenation of saturated C–H bonds by a dimanganese catalyst. Science 312, 1941–1943 (2006).
Phipps, R. J. & Gaunt, M. J. A. meta-selective copper-catalyzed C–H bond arylation. Science 323, 1593–1597 (2009).
Saidi, O. et al. Ruthenium-catalyzed meta sulfonation of 2-phenylpyridines. J. Am. Chem. Soc. 133, 19298–19301 (2011).
Leow, D., Li, G., Mei, T.-S. & Yu, J.-Q. Activation of remote meta-C–H bonds assisted by an end-on template. Nature 486, 518–522 (2012).
Kuninobu, Y., Ida, H., Nishi, M. & Kanai, M. A. meta-selective C–H borylation directed by a secondary interaction between ligand and substrate. Nat. Chem. 7, 712–717 (2015).
Bag, S. et al. Remote para-C–H functionalization of arenes by a D-shaped biphenyl template-based assembly. J. Am. Chem. Soc. 137, 11888–11891 (2015).
Okumura, S. et al. Para-selective alkylation of benzamides and aromatic ketones by cooperative nickel/aluminum catalysis. J. Am. Chem. Soc. 138, 14699–14704 (2016).
Stephens, D. E. & Larionov, O. V. Recent advances in the C–H-functionalization of the distal positions in pyridines and quinolines. Tetrahedron 71, 8683–8716 (2015).
Berman, A. M., Lewis, J. C., Bergman, R. G. & Ellman, J. A. Rh(i)-catalyzed direct arylation of pyridines and quinolines. J. Am. Chem. Soc. 130, 14926–14927 (2008).
Takagi, J., Sato, K., Hartwig, J. F., Ishiyama, T. & Miyaura, N. Iridium-catalyzed C–H coupling reaction of heteroaromatic compounds with bis(pinacolato)diboron: regioselective synthesis of heteroarylboronates. Tetrahedron Lett. 43, 5649–5651 (2002).
Ye, M., Gao, G.-L. & Yu, J.-Q. Ligand-promoted C-3 selective C–H olefination of pyridines with Pd catalysts. J. Am. Chem. Soc. 133, 6964–6967 (2011).
Nakao, Y., Yamada, Y., Kashihara, N. & Hiyama, T. Selective C-4 alkylation of pyridine by nickel/Lewis acid catalysis. J. Am. Chem. Soc. 132, 13666–13668 (2010).
Tsai, C.-C. et al. Bimetallic nickel aluminun mediated para-selective alkenylation of pyridine: direct observation of η 2,η 1-pyridine Ni(0)-Al(iii) intermediates prior to C–H bond activation. J. Am. Chem. Soc. 132, 11887–11889 (2010).
Yamamoto, S., Saga, Y., Andou, T., Matsunaga, S. & Kanai, M. Cobalt-catalyzed C-4 selective alkylation of quinolines. Adv. Synth. Catal. 356, 401–405 (2014).
Kwak, J., Kim, M. & Chang, S. Rh(NHC)-catalyzed direct and selective arylation of quinolines at the 8-position. J. Am. Chem. Soc. 133, 3780–3783 (2011).
Konishi, S. et al. Site-selective C−H borylation of quinolines at the C8 position catalyzed by a silica-supported phosphane-iridium system. Chem. Asian J. 9, 434–438 (2014).
Zhang, Z., Tanaka, K. & Yu, J.-Q. Remote site-selective C–H activation directed by a catalytic bifunctional template. Nature 543, 538–542 (2017).
Wang, X.-C. et al. Ligand-enabled meta-C–H activation using a transient mediator. Nature 519, 334–338 (2015).
Dong, Z., Wang, J. & Dong, G. Simple amine-directed meta-selective C–H arylation via Pd/norbornene catalysis. J. Am. Chem. Soc. 137, 5887–5890 (2015).
Shen, P.-X., Wang, X.-C., Wang, P., Zhu, R.-Y. & Yu, J.-Q. Ligand-enabled meta-C–H alkylation and arylation using a modified norbornene. J. Am. Chem. Soc. 137, 11574–11577 (2015).
Ye, J. & Lautens, M. Palladium-catalysed norbornene-mediated C−H functionalization of arenes. Nat. Chem. 7, 863–870 (2015).
Della, Ca’N., Fontana, M., Motti, E. & Catellani, M. Pd/norbornene: a winning combination for selective aromatic functionalization via C–H bond activation. Acc. Chem. Res. 49, 1389–1400 (2016).
Shi, H., Herron, A. N., Shao, Y., Shao, Q. & Yu, J.-Q. Enantioselective remote meta-C–H arylation and alkylation via a chiral transient mediator. Nature 558, 581–586 (2018).
Wang, P. et al. Ligand-accelerated non-directed C–H functionalization of arenes. Nature 551, 489–493 (2017).
Bracher, F. & Tremmel, T. From lead to drug utilizing a Mannich reaction: the topotecan story. Arch. Pharm. Chem. Life Sci. 350, e1600236 (2017).
Acknowledgements
We acknowledge Scripps Research, the NIH (National Institute of General Medical Sciences grant no. R01GM102265), the National Science Foundation (NSF, CHE-1764328 to K.N.H.) and the CCI Center for Selective C–H Functionalization (CHE-1700982 to K.N.H) for financial support. Computational studies were also supported by the NSF (OCI-1053575 to K.N.H.). Y.L. and J.W. thank the China Scholarship Council for support.
Author information
Authors and Affiliations
Contributions
J.-Q.Y. and H.S. conceived the concept. H.S. developed the remote site-selective arylation of benzoazines. H.S. and Y.L. developed the remote site-selective arylation of arenes. H.S., Y.L., J.W. and K.T. prepared templates and reaction substrates. P.V., K.L.B., X.C. and K.N.H. performed the density functional theory calculations. J.-Q.Y. directed the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary experimental details, compound characterization data, Supplementary tables, Supplementary figure, computational study.
Crystallographic data
Crystallographic data for compound 2ah. CCDC reference 1890836.
Rights and permissions
About this article
Cite this article
Shi, H., Lu, Y., Weng, J. et al. Differentiation and functionalization of remote C–H bonds in adjacent positions. Nat. Chem. 12, 399–404 (2020). https://doi.org/10.1038/s41557-020-0424-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-0424-5
This article is cited by
-
Regioselective umpolung para-C–H functionalization of arylhydroxylamines
Nature Synthesis (2023)
-
Tunably strained metallacycles enable modular differentiation of aza-arene C–H bonds
Nature Communications (2023)
-
Expanding chemical space by para-C−H arylation of arenes
Nature Communications (2022)
-
Molecular editing of aza-arene C–H bonds by distance, geometry and chirality
Nature (2022)
-
A directive Ni catalyst overrides conventional site selectivity in pyridine C–H alkenylation
Nature Chemistry (2021)