Abstract
Objectives
The aim of this study was to evaluate the diagnostic performance of introducing diffusion-weighted imaging (DWI) as a major feature to extracellular agent (ECA)-MRI for diagnosing HCC in comparison with gadoxetic acid (hepatobiliary agent, HBA)-MRI using Liver Imaging Reporting and Data System (LI-RADS) v2018.
Methods
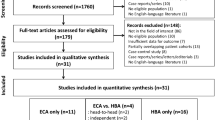
This was a prospective intra-individual comparison study using two different types of contrast agents for liver MRI conducted at a tertiary referral academic center. One hundred forty-seven observations in 122 patients at high risk for HCC scheduled for liver surgery were included. The sensitivity, specificity, and accuracy of LI-RADS category 5 (LR-5) for HCC diagnosis according to conventional and modified LI-RADS on ECA- and HBA-MRI were measured and compared. Modified LI-RADS incorporated hyperintensity on DWI as a major feature with ECA-MRI, and hypointensity on transitional phase (TP) and/or hepatobiliary phase (HBP) as washout appearance on HBA-MRI, respectively.
Results
Modified LI-RADS on ECA-MRI had higher sensitivity and accuracy than modified LI-RADS on HBA-MRI (90.3% vs. 74.9%, p < 0.001; and 91.9% vs. 76.9%, p < 0.001, respectively), as well as higher specificity, although the difference did not reach statistical significance (96.0% vs. 88.0%, p = 0.157). The specificity of modified LI-RADS ECA-MRI was slightly lower than both conventional criteria but without a significant difference (96.0% vs. 100%, p = 0.317).
Conclusions
Using DWI findings as a major feature for modified LR-5 on ECA-MRI showed better sensitivity and accuracy than modified LR-5 on HBA-MRI, without significantly compromising specificity compared with conventional LR-5 on ECA- or HBA-MRI.
Key Points
• Prospective intra-individual comparison study using two different types of contrast agents, extracellular agent (ECA) and gadoxetic acid (hepatobiliary agent, HBA), for liver MRI was conducted.
• Applying diffusion restriction of a hepatic observation on ECA-MRI as a major feature of LI-RADS v2018 resulted in higher sensitivity and accuracy of LR-5 observations for HCC diagnosis than conventional LI-RADS v2018, and even compared to modified LI-RADS using modified washout on HBA-MRI.
• Despite increase in sensitivity and accuracy of LR-5 observations on modified LI-RADS on ECA-MRI, the specificity was not significantly different compared with conventional LI-RADS.




Similar content being viewed by others
Abbreviations
- AASLD:
-
American Association for the Study of Liver Disease
- APHE:
-
Arterial phase hyperenhancement
- APS:
-
Arterioportal shunt
- CHC:
-
Combined hepatocellular-cholangiocarcinomas
- CT:
-
Computed tomography
- DN:
-
Dysplastic nodules
- DP:
-
Delayed phase
- DWI:
-
Diffusion-weighted imaging
- EASL:
-
European Association for the Study of the Liver
- ECA:
-
Extracellular agent
- HBP:
-
Hepatobiliary phase
- HBA:
-
Hepatobiliary agent
- HCC:
-
Hepatocellular carcinoma
- HH:
-
Hepatic hemangioma
- ICC:
-
Intrahepatic cholangiocarcinoma
- LI-RADS:
-
Liver Imaging Reporting and Data System
- LR:
-
LI-RADS category
- LT:
-
Liver transplantation
- mLR:
-
Modified LI-RADS category
- MRI:
-
Magnetic resonance imaging
- NET:
-
Neuroendocrine tumor
- NP:
-
Negative predictive value
- PPV:
-
Positive predictive value
- PVP:
-
Portal venous phase
- TP:
-
Transitional phase
References
American College of Radiology. Liver Imaging Reporting and Data System. Available via http://www.acr.org/Quality-Safety/Resources/LIRADS. Accessed August 20, 2018
European Association for the Study of the Liver (2018) EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 69:182–236
Forner A, Vilana R, Ayuso C et al (2008) Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 47:97–104
Choi JY, Lee JM, Sirlin CB (2014) CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology 273:30–50
Joo I, Lee JM, Lee DH, Jeon JH, Han JK (2019) Retrospective validation of a new diagnostic criterion for hepatocellular carcinoma on gadoxetic acid-enhanced MRI: can hypointensity on the hepatobiliary phase be used as an alternative to washout with the aid of ancillary features? Eur Radiol 29:1724–1732
Ahn SS, Kim MJ, Lim JS, Hong HS, Chung YE, Choi JY (2010) Added value of gadoxetic acid-enhanced hepatobiliary phase MR imaging in the diagnosis of hepatocellular carcinoma. Radiology 255:459–466
Choi SH, Byun JH, Lim YS et al (2016) Diagnostic criteria for hepatocellular carcinoma 3 cm with hepatocyte-specific contrast-enhanced magnetic resonance imaging. J Hepatol 64:1099–1107
Cha DI, Lee MW, Kim YK et al (2014) Assessing patients with hepatocellular carcinoma meeting the Milan criteria: is liver 3 tesla MR with gadoxetic acid necessary in addition to liver CT? J Magn Reson Imaging 39:842–852
Kim HD, Lim YS, Han S et al (2015) Evaluation of early-stage hepatocellular carcinoma by magnetic resonance imaging with gadoxetic acid detects additional lesions and increases overall survival. Gastroenterology 148:1371–1382
Narita M, Hatano E, Arizono S et al (2009) Expression of OATP1B3 determines uptake of Gd-EOB-DTPA in hepatocellular carcinoma. J Gastroenterol 44:793–798
Taouli B (2012) Diffusion-weighted MR imaging for liver lesion characterization: a critical look. Radiology 262:378–380
Piana G, Trinquart L, Meskine N, Barrau V, Beers BV, Vilgrain V (2011) New MR imaging criteria with a diffusion-weighted sequence for the diagnosis of hepatocellular carcinoma in chronic liver diseases. J Hepatol 55:126–132
Xu PJ, Yan FH, Wang JH, Lin J, Ji Y (2009) Added value of breathhold diffusion-weighted MRI in detection of small hepatocellular carcinoma lesions compared with dynamic contrast-enhanced MRI alone using receiver operating characteristic curve analysis. J Magn Reson Imaging 29:341–349
Cha DI, Jang KM, Kim SH, Kang TW, Song KD (2017) Liver imaging reporting and data system on CT and gadoxetic acid-enhanced MRI with diffusion-weighted imaging. Eur Radiol 27:4394–4405
Sandrasegaran K, Tahir B, Patel A et al (2013) The usefulness of diffusion-weighted imaging in the characterization of liver lesions in patients with cirrhosis. Clin Radiol 68:708–715
Min JH, Kim JM, Kim YK et al (2018) Prospective intraindividual comparison of magnetic resonance imaging with gadoxetic acid and extracellular contrast for diagnosis of hepatocellular carcinomas using the liver imaging reporting and data system. Hepatology 68:2254–2266
Song JS, Choi EJ, Hwang SB, Hwang HP, Choi H (2019) LI-RADS v2014 categorization of hepatocellular carcinoma: intraindividual comparison between gadopentetate dimeglumine-enhanced MRI and gadoxetic acid-enhanced MRI. Eur Radiol 29:401–410
Schneider G, Maas R, Schultze Kool L et al (2003) Low-dose gadobenate dimeglumine versus standard dose gadopentetate dimeglumine for contrast-enhanced magnetic resonance imaging of the liver: an intra-individual crossover comparison. Invest Radiol 38:85–94
Kuwatsuru R, Kadoya M, Ohtomo K et al (2001) Comparison of gadobenate dimeglumine with gadopentetate dimeglumine for magnetic resonance imaging of liver tumors. Invest Radiol 36:632–641
Bossuyt PM, Reitsma JB, Bruns DE et al (2015) STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. Radiology 277:826–832
Taouli B, Koh DM (2010) Diffusion-weighted MR imaging of the liver. Radiology 254:47–66
Park HJ, Kim YK, Park MJ, Lee WJ (2013) Small intrahepatic mass-forming cholangiocarcinoma: target sign on diffusion-weighted imaging for differentiation from hepatocellular carcinoma. Abdom Imaging 38:793–801
Connor RJ (1987) Sample size for testing differences in proportions for the paired-sample design. Biometrics:207–211
Bruegel M, Holzapfel K, Gaa J et al (2008) Characterization of focal liver lesions by ADC measurements using a respiratory triggered diffusion-weighted single-shot echo-planar MR imaging technique. Eur Radiol 18:477–485
Parikh T, Drew SJ, Lee VS et al (2008) Focal liver lesion detection and characterization with diffusion-weighted MR imaging: comparison with standard breath-hold T2-weighted imaging. Radiology 246:812–822
Nakamura Y, Toyota N, Date S et al (2011) Clinical significance of the transitional phase at gadoxetate disodium-enhanced hepatic MRI for the diagnosis of hepatocellular carcinoma: preliminary results. J Comput Assist Tomogr 35:723–727
Cereser L, Furlan A, Bagatto D et al (2010) Comparison of portal venous and delayed phases of gadolinium-enhanced magnetic resonance imaging study of cirrhotic liver for the detection of contrast washout of hypervascular hepatocellular carcinoma. J Comput Assist Tomogr 34:706–711
Okamoto D, Yoshimitsu K, Nishie A et al (2012) Enhancement pattern analysis of hypervascular hepatocellular carcinoma on dynamic MR imaging with histopathological correlation: validity of portal phase imaging for predicting tumor grade. Eur J Radiol 81:1116–1121
Tamada T, Ito K, Sone T et al (2009) Dynamic contrast-enhanced magnetic resonance imaging of abdominal solid organ and major vessel: comparison of enhancement effect between Gd-EOB-DTPA and Gd-DTPA. J Magn Reson Imaging 29:636–640
Ringe KI, Husarik DB, Sirlin CB, Merkle EM (2010) Gadoxetate disodium-enhanced MRI of the liver: part 1, protocol optimization and lesion appearance in the noncirrhotic liver. AJR Am J Roentgenol 195:13–28
Cha DI, Kang TW, Jang KM et al (2018) Hepatic neuroendocrine tumors: gadoxetic acid-enhanced magnetic resonance imaging findings with an emphasis on differentiation between primary and secondary tumors. Abdom Radiol (NY) 43:3331–3339
Chen Z, Xiao HE, Ramchandra P, Huang HJ (2014) Imaging and pathological features of primary hepatic neuroendocrine carcinoma: an analysis of nine cases and review of the literature. Oncol Lett 7:956–962
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Young Kon Kim.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in a previous journal. The patient population of this study is part of a prospective-registry of patients scheduled for surgery gathered in our medical center, and includes partial data from a previous prospective study which included 91 patients (Hepatology 2018:68:2254-2256). This study differs from the previous one in that this study emphasizes on the value of diffusion restriction, an ancillary feature of LI-RADS, on ECA-MRI, whereas the previous one focuses on the value of modified (illusional) washout.
Methodology
• Prospective
• Diagnostic or prognostic study
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1099 kb)
Rights and permissions
About this article
Cite this article
Cha, D.I., Choi, G.S., Kim, Y.K. et al. Extracellular contrast-enhanced MRI with diffusion-weighted imaging for HCC diagnosis: prospective comparison with gadoxetic acid using LI-RADS. Eur Radiol 30, 3723–3734 (2020). https://doi.org/10.1007/s00330-020-06753-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-06753-5