Abstract
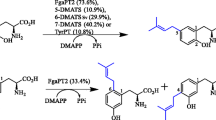
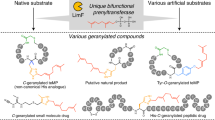
Prenyltransferase NovQ is a vital class involved in the biosynthesis of secondary metabolites such as clorobiocin and novobiocin. To investigate the relationship between structure and catalytic properties of NovQ, here, we have analyzed the substrate-binding site, namely PT barrel, and revealed that menadione hydroquinol formed intermolecular interactions with the residue Glu281 near the center of the active pocket. In this study, Glu281 was substituted with 9 diverse amino acids and catalytic properties of mutants were observed in vitro. Among them, E281Q showed 2.05-fold activities towards the aromatic substrate and prenyl donor, while others obtained catalytic efficiency between 8.4 and 88.6% of that of wild-type NovQ. Furthermore, the effects of catalytic conditions and substrate status on the activity of NovQ and its mutants were considered to obtain the optimized prenylated reaction. When the evolutionary NovQ variant E281Q was overexpressed in the host constructed to synthesize dimethylallyl diphosphate through the engineered mevalonate (MVA) pathway, we harvested up to 4.7 mg/L prenylated menadione at C-3 position by exogenously supplying the aromatic substrate. The construction of the microbial platform based on NovQ opens a new orientation to further biosynthesize various vitamin K2 with other ABBA prenyltransferases in E. coli.





Similar content being viewed by others
Change history
14 April 2020
The published online version contains some errors.
References
Aghaeepoor M, Akbarzadeh A, Mirzaie S, Hadian A, Jamshidi Aval S, Dehnavi E (2018) Selective reduction in glutaminase activity of l-Asparaginase by asparagine 248 to serine mutation: a combined computational and experimental effort in blood cancer treatment. Int J Biol Macromol 120:2448–2457
Araya-Cloutier C, Martens B, Schaftenaar G, Leipoldt F, Gruppen H, Vincken JP (2017) Structural basis for non-genuine phenolic acceptor substrate specificity of Streptomyces roseochromogenes prenyltransferase CloQ from the ABBA/PT-barrel superfamily. PLoS One 12(3):e0174665
Backhaus K, Ludwig-Radtke L, Xie X, Li S-M (2017) Manipulation of the precursor supply in yeast significantly enhances the accumulation of prenylated β-carbolines. ACS Synth Biol 6(6):1056–1064
Bayse CA, Merz KM (2014) Mechanistic insights into Mg2+-independent prenylation by CloQ from classical molecular mechanics and hybrid quantum mechanics/molecular mechanics molecular dynamics simulations. Biochemistry 53(30):5034–5041
Brockmeyer K, Li SM (2017) Mutations of residues in pocket P1 of a cyclodipeptide synthase strongly increase product formation. J Nat Prod 80(11):2917–2922
Chen H, Christopher TW (2001) Coumarin formation in novobiocin biosynthesis: L-hydroxylation of the aminoacyl enzyme tyrosyl-S-NovH by a cytochrome P450 NovI. Chem Biol 8(4):301–312
Haagen Y, Unsold I, Westrich L, Gust B, Richard SB, Noel JP, Heide L (2007) A soluble, magnesium-independent prenyltransferase catalyzes reverse and regular C-prenylations and O-prenylations of aromatic substrates. FEBS Lett 581(16):2889–2893
He C, Ohnishi K (2017) Efficient renaturation of inclusion body proteins denatured by SDS. Biochem Biophys Res Commun 490(4):1250–1253
He B-B, Bu X-L, Zhou T, Li S-M, Xu M-J, Xu J (2018) Combinatory biosynthesis of prenylated 4-hydroxybenzoate derivatives by overexpression of the substrate-promiscuous prenyltransferase XimB in engineered E. coli. ACS Synth Biol 7(9):2094–2104
Kim L, Brudzynski K (2018) Identification of menaquinones (vitamin K2 homologues) as novel constituents of honey. Food Chem 249:184–192
Kong MK, Lee PC (2011) Metabolic engineering of menaquinone-8 pathway of Escherichia coli as a microbial platform for vitamin K production. Biotechnol Bioeng 108(8):1997–2002
Kumano T, Tomita T, Nishiyama M, Kuzuyama T (2010) Functional characterization of the promiscuous prenyltransferase responsible for furaquinocin biosynthesis: identification of a physiological polyketide substrate and its prenylated reaction products. J Biol Chem 285(51):39663–39671
Kuzuyama T (2017) Biosynthetic studies on terpenoids produced by Streptomyces. J Antibiot 70(7):811–818
Kuzuyama T, Noel JP, Richard SB (2005) Structural basis for the promiscuous biosynthetic prenylation of aromatic natural products. Nature 435(7044):983–987
Li W (2016) Bringing bioactive compounds into membranes: the ubiA superfamily of intramembrane aromatic prenyltransferases. Trends Biochem Sci 41(4):356–370
Li Z, Zhao G, Liu H, Guo Y, Wu H, Sun X, Wu X, Zheng Z (2017) Biotransformation of menadione to its prenylated derivative MK-3 using recombinant Pichia pastoris. J Ind Microbiol Biotechnol 44(7):973–985
Mahdinia E, Demirci A, Berenjian A (2018a) Enhanced vitamin K (Menaquinone-7) production by Bacillus subtilis natto in biofilm reactors by optimization of glucose-based medium. Curr Pharm Biotechnol 19(11):917–924
Mahdinia E, Demirci A, Berenjian A (2018b) Implementation of fed-batch strategies for vitamin K (menaquinone-7) production by Bacillus subtilis natto in biofilm reactors. Appl Microbiol Biotechnol 102(21):9147–9157
Mai P, Zocher G, Stehle T, Li SM (2018) Structure-based protein engineering enables prenyl donor switching of a fungal aromatic prenyltransferase. Org Biomol Chem 16(40):7461–7469
Metzger U, Keller S, Stevenson CE, Heide L, Lawson DM (2010) Structure and mechanism of the magnesium-independent aromatic prenyltransferase CloQ from the clorobiocin biosynthetic pathway. J Mol Biol 404(4):611–626
Mori T, Zhang L, Awakawa T, Hoshino S, Okada M, Morita H, Abe I (2016) Manipulation of prenylation reactions by structure-based engineering of bacterial indolactam prenyltransferases. Nat Commun 7(1):10849
Ni W, Liu H, Wang P, Wang L, Sun X, Wang H, Zhao G, Zheng Z (2018) Evaluation of multiple fused partners on enhancing soluble level of prenyltransferase NovQ in Escherichia coli. Bioprocess Biosyst Eng 42(3):465–474
Ozaki T, Mishima S, Nishiyama M, Kuzuyama T (2009) NovQ is a prenyltransferase capable of catalyzing the addition of a dimethylallyl group to both phenylpropanoids and flavonoids. J Antibiot 62(7):385–392
P L, C S-D (2002) Metabolic engineering towards biotechnological production of carotenoids in microorganisms. Appl Microbiol Biotechnol 60(1–2):1–11
Pitera DJ, Paddon CJ, Newman JD, Keasling JD (2007) Balancing a heterologous mevalonate pathway for improved isoprenoid production in Escherichia coli. Metab Eng 9(2):193–207
Pojer F, Wemakor E, Kammerer B, Chen H, Walsh CT, Li SM, Heide L (2003) CloQ, a prenyltransferase involved in clorobiocin biosynthesis. Proc Natl Acad Sci USA 100(5):2316–2321
Qian S, Clomburg JM, Gonzalez R (2019) Engineering Escherichia coli as a platform for the in vivo synthesis of prenylated aromatics. Biotechnol Bioeng 116(5):1116–1127
Redding-Johanson AM, Batth TS, Chan R, Krupa R, Szmidt HL, Adams PD, Keasling JD, Lee TS, Mukhopadhyay A, Petzold CJ (2011) Targeted proteomics for metabolic pathway optimization: application to terpene production. Metab Eng 13(2):194–203
Saleh O, Haagen Y, Seeger K, Heide L (2009) Prenyl transfer to aromatic substrates in the biosynthesis of aminocoumarins, meroterpenoids and phenazines: the ABBA prenyltransferase family. Phytochemistry 70(15–16):1728–1738
Saranya R, Jayapriya J, Tamil Selvi A (2018) Purification, characterization, molecular modeling and docking study of fish waste protease. Int J Biol Macromol 118:569–583
Schumacher MM, Elsabrouty R, Seemann J, Jo Y, DeBose-Boyd RA (2015) The prenyltransferase UBIAD1 is the target of geranylgeraniol in degradation of HMG CoA reductase. Elife 4:1–21
Tello M, Kuzuyama T, Heide L, Noel JP, Richard SB (2008) The ABBA family of aromatic prenyltransferases: broadening natural product diversity. Cell Mol Life Sci 65(10):1459–1463
Wang R, Chen R, Li J, Liu X, Xie K, Chen D, Peng Y, Dai J (2016) Regiospecific prenylation of hydroxyxanthones by a plant flavonoid prenyltransferase. J Nat Prod 79(8):2143–2147
Wang H, Sun X, Wang L, Wu H, Zhao G, Liu H, Wang P, Zheng Z (2018) Coproduction of menaquinone-7 and nattokinase by Bacillus subtilis using soybean curd residue as a renewable substrate combined with a dissolved oxygen control strategy. Ann Microbiol 68(10):655–665
Wei H, Wang L, Zhao G, Fang Z, Wu H, Wang P, Zheng Z (2018a) Extraction, purification and identification of menaquinones from Flavobacterium meningosepticum fermentation medium. Process Biochem 66:245–253
Wei H, Zhao G, Liu H, Wang H, Ni W, Wang P, Zheng Z (2018b) A simple and efficient method for the extraction and separation of menaquinone homologs from wet biomass of Flavobacterium. Bioprocess Biosyst Eng 41(1):107–113
Xie F, Zhang W, Gong S, Gu X, Lan X, Wu J, Wang Z (2019) Investigating lignin from Canna edulis ker residues induced activation of α-amylase: kinetics, interaction, and molecular docking. Food Chem 271:62–69
Yang Y, Miao Y, Wang B, Cui G, Merz KM (2012) Catalytic mechanism of aromatic prenylation by NphB. Biochemistry 51(12):2606–2618
Yang J, Wang Q, Zhou Y, Li J, Gao R, Guo Z (2017) Engineering T. naphthophila β-glucosidase for enhanced synthesis of galactooligosaccharides by site-directed mutagenesis. Biochem Eng J 127:1–8
Zhang X-J, Deng H-Z, Liu N, Gong Y-C, Liu Z-Q, Zheng Y-G (2019) Molecular modification of a halohydrin dehalogenase for kinetic regulation to synthesize optically pure (S)-epichlorohydrin. Bioresour Technol 276:154–160
Zhao W, Fan A, Tarcz S, Zhou K, Yin WB, Liu XQ, Li SM (2017) Mutation on Gly115 and Tyr205 of the cyclic dipeptide C2-prenyltransferase FtmPT1 increases its catalytic activity toward hydroxynaphthalenes. Appl Microbiol Biotechnol 101(5):1989–1998
Zirpel B, Degenhardt F, Martin C, Kayser O, Stehle F (2017) Engineering yeasts as platform organisms for cannabinoid biosynthesis. J Biotechnol 259:204–212
Funding
This work was supported by the Key research and development plan of Anhui Province (1804b06020342), Natural Science Foundation of Anhui Province (1908085MB48 and 1908085MB43), China National Key Research and Development Program (2019YFA0904300) and Major Projects of Science and Technology in Anhui Province (17030801036).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: The published online version contains an error for Figure 1. The corrected figure is in this version.
Electronic supplementary material
ESM 1
(PDF 446 kb)
Rights and permissions
About this article
Cite this article
Ni, W., Zheng, Z., Liu, H. et al. Combining mutagenesis on Glu281 of prenyltransferase NovQ and metabolic engineering strategies for the increased prenylated activity towards menadione. Appl Microbiol Biotechnol 104, 4371–4382 (2020). https://doi.org/10.1007/s00253-020-10470-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10470-w