Abstract
Background
Mechanical ventilation of preterm neonates is associated with neuroinflammation and an increased risk of adverse neurological outcomes. Human amnion epithelial cells (hAECs) have anti-inflammatory and regenerative properties. We aimed to determine if intravenous administration of hAECs to preterm lambs would reduce neuroinflammation and injury at 2 days of age.
Methods
Preterm lambs were delivered by cesarean section at 128−130 days’ gestation (term is ~147 days) and either ventilated for 48 h or humanely killed at birth. Lambs received 3 mL surfactant (Curosurf) via endotracheal tube prior to delivery (either with or without 90 × 106 hAECs) and 3 mL intravenous phosphate-buffered saline (with or without 90 × 106 hAECs, consistent with intratracheal treatment) after birth.
Results
Ventilation increased microglial activation, total oligodendrocyte cell number, cell proliferation and blood−brain barrier permeability (P < 0.05, PBS + ventilation and hAEC + ventilation vs. control), but did not affect numbers of immature and mature oligodendrocytes. Ventilation reduced astrocyte and neuron survival (P < 0.05, PBS + ventilation and hAEC + ventilation vs. control). hAEC administration did not alter markers of neuroinflammation or injury within the white or gray matter.
Conclusions
Mechanical ventilation for 48 h upregulated markers of neuroinflammation and injury in preterm lambs. Administration of hAECs did not affect markers of neuroinflammation or injury.
Impact
-
Mechanical ventilation of preterm lambs for 48 h, in a manner consistent with contemporary neonatal intensive care, causes neuroinflammation, neuronal loss and pathological changes in oligodendrocyte and astrocyte survival consistent with evolving neonatal brain injury.
-
Intravenous administration of hAECs immediately after birth did not affect neonatal cardiorespiratory function and markers of neuroinflammation or injury.
-
Reassuringly, our findings in a translational large animal model demonstrate that intravenous hAEC administration to the preterm neonate is safe.
-
Considering that hAECs are being used in phase 1 trials for the treatment of BPD in preterm infants, with future trials planned for neonatal neuroprotection, we believe these observations are highly relevant.
Similar content being viewed by others
Introduction
Mechanical ventilation of preterm neonates is lifesaving but can cause inadvertent neuroinflammation and injury. Mechanical ventilation using high tidal volumes (VT) may cause systemic and cerebral inflammation, impaired cerebral perfusion and cerebrovascular injury1,2. Furthermore, delivery of VT ventilation within a safe range is less injurious than high VT ventilation, but is still associated with brain inflammation and injury1. Regardless of the ventilation strategy, mechanical ventilation is associated with an increased risk of brain injury and poor neurodevelopmental outcomes after preterm birth3,4. In preterm human and animal studies, injury is commonly characterized by diffuse gliosis and oligodendrocyte cell death5,6,7 which in severe cases is accompanied by neuronal27 and cerebrovascular injury2. However, gliosis and myelination deficits without oligodendrocyte loss have also been reported in human infants8. This inflammation and injury may be amenable to therapy to improve outcomes in preterm infants.
Human amnion epithelial cells (hAECs) possess immunomodulatory9 and reparative characteristics10. Extracted from the lining of the inner layer of the placenta, hAECs are readily obtainable and have low tumorigenicity11. They have a low immunogenic profile because they express low levels of the human leukocyte antigen allowing allogeneic transplantation without the need for immune suppression. Systemic hAEC administration attenuated fetal lung and brain inflammation after systemic or intra-amniotic lipopolysaccharide injection in preclinical studies12,13. Intranasal hAEC administration reduced neuroinflammation and improved myelination in a preterm fetal sheep model of moderate-severe hypoxic-ischemic encephalopathy14. hAECs decreased microglial density and improved blood−brain barrier integrity, but elevated levels of proinflammatory cytokines in the white matter within 2 h after birth in preterm lambs ventilated with high VT 15. However, longer postnatal survival times have not been evaluated in large animal studies. Given that perinatal brain injury can evolve for days to weeks after birth16, evaluating postnatal outcomes after longer survival times is essential to improving our understanding of the pathophysiology of injury and to determine the efficacy of potential therapeutic interventions.
The aim of this study was to evaluate neonatal white and gray matter brain injury after 48 h of mechanical ventilation, and to determine whether hAEC administration at birth could reduce inflammation and promote cell survival in the brains of preterm lambs.
Methods
Human amnion epithelial cell isolation and preparation
All procedures relating to hAEC isolation and preparation were approved by the Monash Health Research Ethics Committee (approval no. 12223B). Placentae were obtained from uncomplicated term pregnancies, delivered by elective cesarean section at Monash Medical Centre. We used multiple donors to ensure that the efficacy and potency of the cells administered was maximized. The amniotic membrane was separated from the chorion and hAECs were isolated via enzymatic treatment with two sequential incubations in 0.05% trypsin-EDTA (Gibco, Invitrogen, Australia) at 37 °C for 60 min with vigorous agitation. The supernatant was collected and newborn calf serum was used to inactivate trypsin-EDTA. Supernatant was centrifuged for 10 min at 1800 × g at room temperature before resuspension in Dulbeccoʼs Modified Eagle Medium (DMEM)/F12 media supplemented with 10% FBS. Trypan blue exclusion was used to assess cell viability and number prior to cryopreservation in fetal bovine serum (FBS) with 5% dimethyl sulfoxide in liquid nitrogen. Immediately before administration, cells were thawed in a 37 °C water bath and supplemented with DMEM/F12 media. Cell counts and viability were reassessed before hAECs were resuspended (30 × 106 cells/mL) in sterile phosphate-buffered saline (PBS) for administration. hAECs are positive for epithelial cell adhesion molecule but do not express CD31, 45, 90 and 10517. Cell isolates with a minimum of 80% post-thaw viability were used.
Subjects
The UWA Animal Ethics Committee approved all animal experimentation, in accordance with the National Health and Medical Research Council Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (Eighth Edition; Approval RA 3/100/1138)18. Preterm lambs were randomly allocated to undergo 48 h of mechanical ventilation and received two separate doses of hAECs (total 180 × 106 cells; hAEC + Vent; n = 6) or vehicle (PBS + Vent, n = 6) at 128−130 days of gestation (d). A separate group (Control; n = 7) of preterm lambs were humanely killed immediately after delivery.
Experimental protocol
Pregnant ewes of known mating age were housed in individual pens in the University of Western Australia’s Large Animal Facility. Commencing approximately 40 h prior to delivery, pregnant ewes received two intramuscular doses of betamethasone (0.15 mg/kg) at 24-h intervals to induce maturation of the fetal lungs. Ewes were fasted 24 h prior to delivery, drinking water was supplied. At 128−130 days of gestation, an intramuscular dose of Acepromazine (0.01 mg/kg) and Buprenorphine (0.02 mg/kg) was administered to ewes 1 h before the induction of anesthesia by intravenous administration of ketamine (5 mg/kg) and diazepam (0.25 mg/kg). Ewes were intubated and anesthesia was maintained by delivery of 1−2.5% isoflurane via positive pressure ventilation.
The ewe was placed in a supine position and the ventral abdomen was shorn and cleaned with chlorhexidine antiseptic solution, betadine surgical scrub and betadine solution. All surgical utensils, gowns, drapes and towels were sterilized with an autoclave. All maternal and fetal surgical procedures were performed under aseptic conditions. A paramedian abdominal incision was made and the fetal head was exposed through a uterine incision. A polyvinyl catheter was inserted into the jugular vein. The fetus was intubated with a 4.0 mm endotracheal tube and excess lung liquid was drained passively before random allocation to receive intratracheal surfactant (3 mL, 80 mg/mL, poractant alfa, Chielsi Farmaceutici S.p.A.) and either 90 × 106 hAEC (in 3 mL PBS) or vehicle (3 mL PBS). Surfactant exposure does not affect viability or function of hAECs19.
Mechanical ventilation
The umbilical cord was clamped and cut and the lamb was weighed, dried and placed prone, suspended in a sling in a neonatal baby warmer. Body temperature was maintained between 37 and 38 °C throughout the experimental period. Preterm lambs were ventilated with heated humidified gas with an FIO2 0.21, a rate of 50 breaths/min, an inspiratory time of 0.3−0.6 s, positive-end expiratory pressure (PEEP) of 6 cmH2O and a target VT of 5−7 mL/kg. A i.v. dose of either 90 × 106 hAEC (in 3 mL PBS) or vehicle (3 mL PBS) was administered to the lamb via the jugular vein catheter immediately after initiation of ventilation. Intermittent intravenous doses of lorazepam and fentanyl were administered at 2−3 h intervals after delivery for sedation and analgesia. Lambs received an intravenous caffeine load (15 mg/kg) at 1 h, with a maintenance dose (5 mg/kg) administered 24 h later. Penicillin (100,000 U/kg) and gentamicin (2.5 mg/kg) were administered intravenously at 24-h intervals. Blood gas sampling and ventilation monitoring of the lamb was performed at 30-min intervals. Enteral feeds containing colostrum supplement were administered at 2-h intervals via an orogastric tube commencing at 30 mL/kg/d and increasing by 20 mL/kg/d (Impact Colostrum Supplement, Wombaroo Food Products, SA, Australia). Lambs were ventilated for 48 h before being humanely killed with an overdose of pentobarbitone (100 mg/kg; Lethabarb, Virbac Pty Ltd, NSW, Australia).
Histopathology
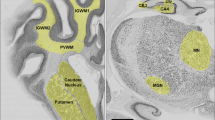
Fetal brains were perfusion fixed in situ with 500 mL of 0.9% saline, followed by 1 L of 10% phosphate-buffered formalin, via gravity perfusion. The skull was removed and brains were fixed for another 5 days. Using a standard paraffin tissue preparation, the right hemisphere was processed and embedded. Using a brain mold, the right hemisphere was cut with a blocking blade into 5-mm-thick coronal blocks. Blocks from the forebrain (approximately 23 mm anterior to stereotaxic zero) with a clearly visible striatum, containing the caudate nucleus, putamen and periventricular and intragyral white matter, were sectioned by a microtome (Leica Jung RM2035, Leica Microsystems, Albany, New Zealand) into 10-μm-thick coronal sections.
Immunohistochemistry
Slides were dewaxed and rehydrated with xylene and decreasing concentrations of ethanol. Antigen retrieval was performed using a microwave technique in citrate buffer. Endogenous peroxidase quenching was performed by incubating slides in 0.1% H2O2 in methanol or PBS (for rabbit anti-sheep serum). Nonspecific antigens were blocked using 3% normal goat serum or 2% fish gelatin in CAS block (for rabbit anti-sheep serum). Sections were labeled with 1:200 rabbit anti-Neuronal nuclei (NeuN) (Abcam, VIC, Australia) to stain neurons, 1:200 rabbit anti-Oligodendrocyte transcription factor-2 (Olig-2) (Abcam, a marker of oligodendrocytes at all stages of development), 1:200 rabbit anti-ionized calcium-binding adaptor molecule 1 (Iba1) (Abcam) to label microglia, 1:200 rabbit anti-Glial fibrillary acidic protein (GFAP) (Abcam) to label astrocytes, 1:200 mouse anti-2′-3′Cyclic-nucleotide 3′-phosphodiesterase (CNPase) (Abcam) to label immature and mature oligodendrocytes, 1:800 rat anti-human Caspase 3 (R&D Systems, VIC, Australia) to stain cells undergoing early apoptosis, 1:200 rabbit anti-Ki67 (Thermo Fisher, VIC, Australia) to stain proliferating cells and 1:2000 rabbit anti-sheep serum (Sigma Aldrich, NSW, Australia) to stain protein extravasation. All primary antibody incubations were performed overnight at 4 °C. The sections were incubated in biotin-conjugated IgG (1:200, goat anti rabbit (Dako, VIC, Australia), rat or mouse (Vector Laboratories, CA, USA)) for 3 h at room temperature before being incubated in avidin−biotin complex (Sigma Aldrich). Sections were allowed to react with 3,3′diaminobenzidine tetrahydrochloride (Sigma Aldrich) as a chromogen. The reaction was ended by washing the slides in PBS. At the end of the staining procedure, slides were dehydrated in xylene and increasing concentrations of ethanol, mounted in dibutylphthalate polystyrene xylene and coverslipped.
Positively stained cells were visualized using light microscopy (Olympus, Tokyo, Japan) at ×40 magnification and cellSens Imaging Software (Version 2.3, Olympus). Images were quantified using ImageJ (NIH, MD, USA). NeuN-positive cells were counted if they were morphologically normal, cells displaying condensed or fragmented nuclei were not counted20. Microglia (Iba-1-positive cells) showing ramified or amoeboid morphology were included in our assessment21. Cleaved caspase-3-positive cells were counted based on morphological assessment, cells displaying both staining and apoptotic bodies were counted.
NeuN-, GFAP-, Iba-1- and Caspase-3-positive cells were quantified from two images captured within each of the putamen and the caudate nucleus and three images within each of the first and second parasagittal gyri. Olig-2-, CNPase-, GFAP-, Iba-1-, Ki67- and Caspase-3-positive cells were quantified from two images in the periventricular white matter and three images within the intragyral white matter tracts from the first and second parasagittal gyri (Fig. 1). Serum extravasation was quantified using cellSense by highlighting the periventricular and intragyral white matter tracts and counting all blood vessels displaying extravasation, as well as the total number of blood vessels in these regions. For each region of interest (Fig. 1), average scores from two slides from the right hemisphere were calculated. All analyses were performed by an assessor (F.N.) who was blinded to the treatment group.
Statistical analysis
Statistical analysis was completed using GraphPad Prism (version 7.00 for Mac, GraphPad Software, La Jolla, California, USA). Between-group comparisons of cardiorespiratory and blood gas data were performed using a two-way ANOVA with repeated measures. Between-group comparisons of neuropathological data were performed using a two-way ANOVA with treatment and region as individual factors. The Tukey test was used for post hoc comparisons. Linear regression was used to assess the relationship between cell proliferation (Ki67) and oligodendrocyte (Olig-2) cell number, and neuronal survival (NeuN) and microglial activation (amoeboid microglia). Data are presented as the mean ± standard error of the mean (SEM). Statistical significance was accepted when P < 0.05.
Results
One animal from the hAEC + ventilation group developed a pneumothorax and died during the first 24 h of the experiment. Data from this animal were omitted from analyses. Arterial blood base deficits of −12 to −14 were observed immediately after birth in one subject from the PBS + vent group and one subject from the hAECs + vent group, suggestive of moderate perinatal asphyxia.
Blood gas composition, pH and ventilation parameters
Mean arterial blood pressure, tidal volume (VT), peak inspiratory pressure (PIP), mean airway pressure (PAW) and the fraction of inspired oxygen (FIO2) and rectal (core body) temperature did not differ between groups (Table 1). Preductal arterial pH, partial pressure of arterial carbon dioxide (PaCO2) and oxygen (PaO2), oxygen saturation (SaO2), base excess, blood lactate and glucose concentrations did not differ between groups throughout the study (Table 2).
Post mortem findings
There were no differences in circulating white blood cell concentrations between groups. Body weight, brain weight and the ratio of males to females were not different between groups (Table 3).
Histopathology
The total number of Iba-1-positive microglia did not differ between the ventilated and control groups. However, in the intragyral and periventricular white matter tracts, the proportion of amoeboid to total microglia was higher in the ventilated groups compared to control (P < 0.05, Figs. 2 and 3). Dense patches of microgliosis were observed in the periventricular white matter in four of the ventilated subjects, two from the PBS + vent group and two from the hAEC + vent group. The number of GFAP-positive cells was lower in the PBS + vent and hAECs + vent groups compared to controls in the intragyral and periventricular white matter tracts (P < 0.05, Figs. 2 and 3). The number of GFAP-positive cells did not differ between the ventilated groups (Figs. 2 and 3). GFAP-positive astrocytes displayed an activated phenotype in the ventilated groups, as shown by larger cell bodies with larger, thicker and retracted processes22, compared to controls (Fig. 3).
Left: Iba-1, proportion of (%) activated microglia-, GFAP- and cleaved caspase-3 (CC3)-positive cell counts. Right: Ki-67-, Olig-2- and CNPase-positive cell counts in the intragyral and periventricular white matter tracts (IGWM and PVWM, respectively) in control (n = 7), PBS + ventilation (n = 6) and hAEC + ventilation (n = 5) groups. *P < 0.05 vs. control.
Arrow heads in the Iba-1 photomicrographs indicate microglia displaying a resting ramified phenotype (small cell body with >1 branching process. Arrows in the representative examples from the ventilated groups indicate an amoeboid morphology (large cell bodies, with ≤1 branching process). Arrows in the GFAP photomicrographs indicate GFAP-positive astrocytes; note the large cell bodies with thick retracted processes in the ventilated groups, indicating reactive astrocytosis. Arrows in the CC3 photomicrograph indicate positive cells displaying both staining and apoptotic bodies. Scale bar is 100 µm.
In the intragyral white matter, there were more Olig-2-positive oligodendrocytes in the PBS + vent group compared to control (P < 0.05). In the periventricular white matter (PVWM), there were more Olig-2-positive cells in the PBS + vent and hAEC + vent groups compared to control (P < 0.01, Figs. 2 and 3). The number of immature and mature CNPase-positive oligodendrocytes did not differ between groups within the intragyral and periventricular white matter tracts (Figs. 2 and 3).
The number of Ki67-positive cells in the intragyral and periventricular white matter tracts was greater in the PBS + vent and hAEC + vent groups compared to control (P < 0.05, Figs. 2 and 3). The number of proliferating Ki67-positive cells was not different between the ventilated groups.
The number of cleaved caspase-3-positive cells was greater in the ventilated groups compared to control in the intragyral white matter (P < 0.05, Figs. 2 and 3). In the periventricular white matter, the number of caspase-3-positive cells was not significantly higher in the ventilated groups compared to control.
The total number of vessels with serum extravasation was increased in the periventricular white matter in both ventilated groups compared to control (P < 0.01). There were no differences in serum extravasation between PBS and hAEC-treated ventilated preterm lambs (Fig. 4). Olig-2-positive cell number was positively associated with the number of Ki67-positive cells in the periventricular white matter (linear regression: P < 0.001, r2 = 0.58).
Left: Blood vessel extravasation counts in the intragyral and periventricular white matter tracts (IGWM and PVWM, respectively) in control (n = 7), PBS + ventilation (n = 6) and hAEC + ventilation (n = 5) groups. *P < 0.05 vs. control. Right: representative photomicrographs showing an intact blood vessel with no serum extravasation from a control subject (arrow head) and blood vessels from the ventilated groups (arrows) displaying serum extravasation. Scale bar is 100 µm.
In the cerebral cortex, caudate nucleus and putamen, the total number of Iba-1-positive microglia did not differ between groups. The proportion of amoeboid to total microglia was higher in the ventilated groups compared to control in the cerebral cortex (P < 0.05, Figs. 5 and 6). The proportion of amoeboid to total microglia within the caudate nucleus and putamen did not differ between groups.
Left: Iba-1, proportion of (%) activated microglia- and GFAP-positive cell counts in the cerebral cortex, caudate nucleus and putamen. Right: Cleaved caspase-3- and NeuN-positive cell counts in the cerebral cortex, caudate nucleus and putamen in control (n = 7), PBS + ventilation (n = 6) and hAEC + ventilation (n = 5) groups. *P < 0.05 vs. control.
Scale bar is 100 µm. Arrow heads in the Iba-1 photomicrographs indicate microglia displaying a resting ramified phenotype (small cell body with >1 branching process. Arrows in the representative examples from the ventilated groups indicate an amoeboid morphology (large cell bodies, with ≤1 branching process). Arrows in the CC3 photomicrographs indicate positive cells displaying both staining and apoptotic bodies.
The number of GFAP-positive astrocytes was higher in the PBS+vent group compared to control in the cerebral cortex (P < 0.05). In the hAEC + vent group, the number of GFAP-positive astrocytes in the cortex did not differ from control and PBS + vent groups. In the caudate nucleus and putamen, there were no differences in the number of GFAP-positive astrocytes between groups (P < 0.05, Figs. 5 and 6).
The number of caspase-3-positive cells in the cerebral cortex was higher in the PBS + vent group but not in the hAEC + vent group compared to control (P < 0.05, Figs. 5 and 6). The number of caspase-3-positive cells did not differ between the hAEC + vent and the PBS + vent groups. The number of caspase-3-positive cells in the caudate nucleus and putamen was higher in both of the ventilated groups compared to control (P < 0.05, Figs. 5 and 6).
The number of NeuN-positive cells was lower in the PBS + vent and hAEC + vent groups than control in the cerebral cortex, caudate nucleus and putamen (P < 0.05; Fig. 5). There were no differences in the number of NeuN-positive cells between the ventilated groups. Cortical and striatal (the sum of NeuN-positive cells within the caudate nucleus and putamen for each subject) NeuN-positive cell number correlated negatively with the proportion of activated microglia within the intragyral white matter (linear regression, cortical: P < 0.05, r2 = 0.47, striatal: P < 0.01, r2 = 0.57).
Discussion
Preterm birth and neonatal respiratory support for 2 days elicited neuroinflammation and injury within the white matter and loss of cortical and striatal neurons in late-preterm lambs, which was not affected by administration of hAECs at birth.
Mechanical ventilation of the preterm neonate is associated with neural inflammation, and can worsen outcomes with pre-existing inflammation23. Ventilation of infants born preterm is an antecedent of systemic inflammation within the first 2 h after birth24, likely due to barotrauma and or volutrauma caused by mechanical resuscitation in the delivery room23. Previous studies in preterm lambs identified two key pathways associated with neural inflammation and injury during mechanical ventilation; they include systemic inflammation and destabilization of cardiac output1,21,25. However, the acute nature of these studies has limited our ability to evaluate the evolution of brain injury beyond the immediate neonatal period (the first 2 h after delivery).
In the present study, preterm lambs were mechanically ventilated for 48 h in a manner consistent with contemporary neonatal intensive care. For the first time, we show that mechanical ventilation of preterm lambs for 48 h causes loss of cortical and striatal neurons, as shown by reduced numbers of NeuN-positive neurons and increased numbers of caspase-3-positive cells. However, it is important to consider that loss of NeuN expression may not reflect neuronal loss per se but may also indicate loss of antigenicity due to neuronal injury26. Nevertheless, these data are consistent with observations of cortical and subcortical neuronal injury in mechanically ventilated preterm infants, in whom 40−60% of gray matter injury cases were associated with respiratory distress syndrome27. The augmentation of gray matter abnormalities by white matter inflammation in these preterm infants is consistent with a significant correlation between white matter microglial activation and loss of cortical and striatal neurons, and limited gray matter gliosis in our preterm lambs. By contrast, post mortem studies in preterm human infants have also reported white matter injury without overt neuronal loss. Instead, toll-like receptor 3 activation of subcortical neurons, possibly due to release of damage-associated molecular patterns, was associated with white matter injury28.
We observed white matter inflammation and injury in the form of increased microglial activation, loss of astrocytes and elevated blood−brain barrier permeability within the intragyral and periventricular white matter tracts. Activated microglia contribute to cerebral inflammation and injury through production of cytotoxic substances such as free radicals, proinflammatory cytokines, and enhancement of excitotoxicity29. Furthermore, activated microglia induce reactive astrocytosis, which causes astrocytes to relinquish their trophic and homeostatic functions and instead promote neuronal and glial cell death22. Astrocytic cell death has previously been reported within 48 h of a hypoxic-ischemic insult in neonatal piglets30. In the present study, the reduction in astrocyte survival within the white matter was associated with loss of striatal and cortical neurons after 48 h of recovery, suggesting that astrocytic and neuronal loss during mechanical ventilation are interrelated. These data are supported by in vitro studies showing that neurons are more susceptible to excitotoxicity when cultured in an environment that is deficient in astrocytes31, and promotion of neuronal survival due to glutamate uptake by astrocytes in an excitotoxic environment32.
Breakdown of the blood−brain barrier is associated with brain injury and impaired neurodevelopment33. We observed increased blood−brain barrier permeability in ventilated preterm lambs. The increased microglial activation in the ventilated groups may have contributed to the increase in blood−brain barrier permeability, possibly through glial-derived cytokine infiltration, astrocytic-derived vascular endothelial growth factor A or thymidine phosphorylase production34,35.
Apoptosis, cell proliferation and total oligodendrocyte number were increased in ventilated preterm lambs. The increase in total oligodendrocyte (Olig-2+) number was not a consequence of changes in the proportion of immature and mature myelinating oligodendrocytes, as shown by no differences in CNPase-positive cell number between the ventilated groups and unventilated controls. Furthermore, we observed a significant positive correlation between ki-67- and Olig-2-positive cell number (P < 0.001, r2 = 0.58). Collectively, these data raise the possibility that restorative proliferation of oligodendrocyte progenitors resulted in an increase in the total number of oligodendrocytes, but had no effect on myelination in 2-day-old preterm lambs. A limitation of this study is that for technical reasons we were unable to identify which specific cells expressed caspase 3 or were undergoing cell proliferation. However, these data are highly consistent with landmark studies in preterm humans with diffuse white matter injury, and studies in preterm fetal sheep and neonatal rodents that showed restorative proliferation of oligodendrocytes as early as 24 h after injury5, followed by maturational arrest of oligodendrocytes5,6,36. It is important to acknowledge that developmental white matter injury is complex and may not necessarily involve oligodendrocyte proliferation. For example, white matter gliosis without overt differences in the total number of (Olig-2+) oligodendrocytes has been reported in preterm infants with noncystic periventricular white matter injury37.
The stage of neural maturation of preterm lambs in this study is comparable to that of the term infant38. In relation to oligodendrocyte development, mature oligodendrocytes are the main population likely to be injured at this stage of brain maturation7. Restorative proliferation of oligodendrocytes was reported in studies of acquired brain injury in term equivalent and adult subjects39. However, whether these newly proliferated cells are capable of differentiating into mature oligodendrocytes and myelinating axons remains unknown, and should be the subject of future studies.
Several studies in small and large animal models of perinatal and adult brain injury have reported that systemic and/or local immunomodulation play an integral role in mediating neuroprotective effects of hAECs. For example, hAECs infused into the lungs of late-preterm fetal sheep reduced microglial and astrocyte infiltration during LPS-induced systemic inflammation12,13. Similarly, in a mouse model of autoimmune encephalomyelitis, hAECs suppressed T-cell proliferation and activation, and decreased cytokine production40. hAECs reduced white matter microglial activation, astrocytosis and apoptosis in neonatal mice exposed to inflammation and hyperoxia9. Subsequent in vitro assessment showed that treating LPS-exposed microglia with hAECs reduced expression of proinflammatory M1 microglia and promoted microglial function9. In adult rodents and marmosets, acute and delayed intravenous hAEC administration after experimental stroke reduced neuroinflammation, infarct volume and functional deficits via chemokine receptor modulation41. Our study shows that acute administration of hAECs immediately before and after preterm birth did not alter white and gray matter injury in 2-day-old preterm lambs. We previously showed that administering hAECs to late-preterm lambs was associated with a region-specific reduction in microglial infiltration and improved blood−brain barrier integrity in the white matter after 2 h of mechanical ventilation15. Our observations raise the possibility that the acute reduction in microglial infiltration and improvement in blood−brain barrier permeability observed in preterm lambs that were ventilated for 2 h have resolved by 2 days. Alternatively, the proinjurious, high tidal volume resuscitation protocol used in the previous study compared to the less injurious approach used in this study may have led to discrepancies in the severity of neural inflammation and injury between the studies.
Previously, we showed upregulation of mRNA levels of proinflammatory cytokines (IL-6 and -8) in the periventricular white matter in ventilated lambs after hAEC administration15, raising the possibility that hAECs could augment neuroinflammation and subsequent injury. However, data from our current study show that hAECs did not exacerbate inflammation, cell loss or blood−brain barrier permeability that are commonly associated with overexpression of proinflammatory cytokines23.
We observed no differences in arterial blood pressure, respiratory physiology and blood gas measurements between hAEC-treated preterm lambs compared to vehicle. Collectively, these data demonstrate that hAEC treatment did not affect cardiorespiratory or metabolic function, consistent with previous studies showing no difference in cerebral oxygenation in preterm lambs treated with hAECs15.
At the end of the study, circulating white blood cell counts did not differ between groups. Due to limited blood sampling, we cannot comment on the temporal profile of circulating immune cells in this study. However, the systemic immunomodulatory potential of hAECs has been reviewed in detail by Magatti et al.42. Furthermore, it is well established that hAECs are immune privileged. They do not express HLA class 2 antigens, their use in numerous preclinical and clinical trials has not been associated with immune rejection and first-in-human clinical trials indicate they are safe for use in preterm neonates with established chronic lung disease43.
The chosen dose and route for hAEC administration in this study was based on previous experiments in which hAECs caused regional reductions in the severity of neuroinflammation and brain injury in preterm fetal sheep exposed to intrauterine inflammation12,13, and neonatal lambs exposed to high tidal volume resuscitation15. It is possible that we have not detected subtle improvements in pathological outcomes. However, there was no overall trend to improvements in neuronal survival or reduced white matter inflammation and injury in neonatal lambs treated with hAECs. Therefore, in this experimental setting, it is unlikely that we failed to detect any clinically significant improvements in outcome after 48 h.
In a preterm fetal sheep model of hypoxic-ischemic encephalopathy, delayed and repeated intranasal administration of hAECs was associated with a long-term reduction in neuroinflammation and improvements in neuronal survival, myelination and EEG activity14, suggesting that delayed and repeated doses of hAECs over a longer time period may have resulted in more favorable outcomes in this study. While intratracheal injection of hAECs is associated with improved neural outcomes in preterm fetal sheep exposed to intrauterine inflammation13, speculatively it is possible that intratracheal administration of hAECs to preterm lambs during barotrauma/volutrauma caused by mechanical ventilation may reduce their therapeutic efficacy. Therefore, the route of administration is an important consideration for future studies.
To facilitate neonatal intensive care and survival of preterm lambs to 48 h, the current study included subjects that were closer to term than other studies which investigated the effects of hAECs on preterm fetal brain injury. For example, neural maturation in the 128-d-old lamb (0.87 of gestation) is equivalent to a late-preterm infant whereas the lungs are comparable to an extremely preterm infant between 28 and 30 weeks of gestation38. Brain development is extensive in the latter half of gestation in humans and sheep. The late-preterm lamb has a larger proportion of oligodendrocyte cells in the later, more mature stages of differentiation and the majority of myelination occurs throughout this period7,38. Therefore, given that immature oligodendrocytes are more susceptible to perinatal insults7, it is possible that studies modeling preterm birth and associated brain injury at younger gestational ages in combination with assessment of outcomes after a longer survival time may be more responsive to hAEC treatment.
In conclusion, preterm birth and mechanical ventilation increased histological markers of neuroinflammation and injury in the white and gray matter of preterm lambs managed with contemporary neonatal ventilation strategies. Administration of hAECs at birth did not reduce white or gray matter injury.
References
Polglase, G. R. et al. Initiation of resuscitation with high tidal volumes causes cerebral hemodynamic disturbance, brain inflammation and injury in preterm lambs. PLoS ONE 7, e39535 (2012).
Mian, Q. et al. Impact of delivered tidal volume on the occurrence of intraventricular haemorrhage in preterm infants during positive pressure ventilation in the delivery room. Arch. Dis. Child Fetal Neonatal Ed. 104, F57–F62 (2019).
Walsh, M. C. et al. Extremely low birthweight neonates with protracted ventilation: mortality and 18-month neurodevelopmental outcomes. J. Pediatr. 146, 798–804 (2005).
Loeliger, M. et al. Cerebral outcomes in a preterm baboon model of early versus delayed nasal continuous positive airway pressure. Pediatrics 118, 1640–1653 (2006).
Segovia, K. N. et al. Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann. Neurol. 63, 520–530 (2008).
Buser, J. R. et al. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann. Neurol. 71, 93–109 (2012).
Back, S. A., Riddle, A., Dean, J. & Hohimer, A. R. The instrumented fetal sheep as a model of cerebral white matter injury in the premature infant. Neurotherapeutics 9, 359–370 (2012).
Billiards, S. S. et al. Myelin abnormalities without oligodendrocyte loss in periventricular leukomalacia. Brain Pathol. 18, 153–163 (2008).
Leaw, B. et al. Human amnion epithelial cells rescue cell death via immunomodulation of microglia in a mouse model of perinatal brain injury. Stem Cell Res. Ther. 8, 46 (2017).
Wu, Z., Hui, G., Lu, Y., Wu, X. & Guo, L. Transplantation of human amniotic epithelial cells improves hindlimb function in rats with spinal cord injury. Chin. Med. J. 119, 2101–2107 (2006).
Lim, R. Concise review: fetal membranes in regenerative medicine: new tricks from an old dog? Stem Cells Transl. Med. 6, 1767–1776 (2017).
Yawno, T. et al. Human amnion epithelial cells protect against white matter brain injury after repeated endotoxin exposure in the preterm ovine fetus. Cell Transpl. 26, 541–553 (2017).
Yawno, T. et al. Human amnion epithelial cells reduce fetal brain injury in response to intrauterine inflammation. Dev. Neurosci. 35, 272–282 (2013).
van den Heuij, L. G. et al. Delayed intranasal infusion of human amnion epithelial cells improves white matter maturation after asphyxia in preterm fetal sheep. J. Cereb. Blood Flow Metab. 39, 223–239 (2019).
Barton, S. K. et al. Human amnion epithelial cells modulate ventilation-induced white matter pathology in preterm lambs. Dev. Neurosci. 37, 338–348 (2015).
Galinsky, R. et al. In the era of therapeutic hypothermia, how well do studies of perinatal neuroprotection control temperature? Dev. Neurosci. 39, 7–22 (2017).
Murphy, S. et al. Amnion epithelial cell isolation and characterization for clinical use. Curr. Protoc. Stem Cell Biol. Chapter 1, Unit 1E 6 (2010).
National Health and Medical Research Council (2013) Australian code for the care and use of animals for scientific purposes, 8th edition. Canberra: National Health and Medical Research Council. https://www.nhmrc.gov.au/about-us/publications/australian-code-care-and-use-animals-scientificpurposes#block-views-block-file-attachments-content-block-1
McDonald, C. A., Melville, J. M., Polglase, G. R., Jenkin, G. & Moss, T. J. Maintenance of human amnion epithelial cell phenotype in pulmonary surfactant. Stem Cell Res. Ther. 5, 107 (2014).
Pozo Devoto, V. M., Chavez, J. C., Fiszer & de Plazas, S. Acute hypoxia and programmed cell death in developing CNS: differential vulnerability of chick optic tectum layers. Neuroscience 142, 645–653 (2006).
Stojanovska, V. et al. The effect of antenatal betamethasone on white matter inflammation and injury in fetal sheep and ventilated preterm lambs. Dev. Neurosci. 40, 497–507 (2018).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017).
Galinsky, R. et al. Complex interactions between hypoxia-ischemia and inflammation in preterm brain injury. Dev. Med. Child Neurol. 60, 126–133 (2018).
Bohrer, B., Silveira, R. C., Neto, E. C. & Procianoy, R. S. Mechanical ventilation of newborns infant changes in plasma pro- and anti-inflammatory cytokines. J. Pediatr. 156, 16–19 (2010).
Stojanovska, V. et al. Effects of intrauterine inflammation on cortical gray matter of near-term lambs. Front. Pediatr. 6, 145 (2018).
Unal-Cevik, I., Kilinc, M., Gursoy-Ozdemir, Y., Gurer, G. & Dalkara, T. Loss of NeuN immunoreactivity after cerebral ischemia does not indicate neuronal cell loss: a cautionary note. Brain Res. 1015, 169–174 (2004).
Pierson, C. R. et al. Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathol. 114, 619–631 (2007).
Vontell, R. et al. Cellular mechanisms of toll-like receptor-3 activation in the thalamus are associated with white matter injury in the developing brain. J. Neuropathol. Exp. Neurol. 74, 273–285 (2015).
Czeh, M., Gressens, P. & Kaindl, A. M. The yin and yang of microglia. Dev. Neurosci. 33, 199–209 (2011).
Martin, L. J. et al. Hypoxia ischemia causes abnormalities in glutamate transporters and death of astroglia and neurons in newborn striatum. Ann. Neurol. 42, 335–348 (1997).
Rosenberg, P. A., Amin, S. & Leitner, M. Glutamate uptake disguises neurotoxic potency of glutamate agonists in cerebral cortex in dissociated cell culture. J. Neurosci. 12, 56–61 (1992).
Sugiyama, K., Brunori, A. & Mayer, M. L. Glial uptake of excitatory amino acids influences neuronal survival in cultures of mouse hippocampus. Neuroscience 32, 779–791 (1989).
Wagner, K. et al. Plasma infusions into porcine cerebral white matter induce early edema, oxidative stress, pro-inflammatory cytokine gene expression and DNA fragmentation: implications for white matter injury with increased blood−brain-barrier permeability. Curr. Neurovasc. Res. 2, 149–155 (2005).
Stolp, H. B. et al. Effects of neonatal systemic inflammation on blood−brain barrier permeability and behaviour in juvenile and adult rats. Cardiovasc. Psychiatry Neurol. 2011, 469046 (2011).
Chapouly, C. et al. Astrocytic TYMP and VEGFA drive blood-brain barrier opening in inflammatory central nervous system lesions. Brain 138, 1548–1567 (2015).
Paton, M. C. B. et al. Human umbilical cord blood therapy protects cerebral white matter from systemic LPS exposure in preterm fetal sheep. Dev. Neurosci. 40, 258–270 (2018).
Verney, C. et al. Microglial reaction in axonal crossroads is a hallmark of noncystic periventricular white matter injury in very preterm infants. J. Neuropathol. Exp. Neurol. 71, 251–264 (2012).
Dobbing, J. & Sands, J. Comparative aspects of the brain growth spurt. Early Hum. Dev. 3, 79–83 (1979).
Dewar, D., Underhill, S. M. & Goldberg, M. P. Oligodendrocytes and ischemic brain injury. J. Cereb. Blood Flow Metab. 23, 263–274 (2003).
McDonald, C. A. et al. Immunosuppressive potential of human amnion epithelial cells in the treatment of experimental autoimmune encephalomyelitis. J. Neuroinflammation 12, 112 (2015).
Evans, M. A. et al. Acute or delayed systemic administration of human amnion epithelial cells improves outcomes in experimental stroke. Stroke 49, 700–709 (2018).
Magatti, M., Vertua, E., Cargnoni, A., Silini, A. & Parolini, O. The immunomodulatory properties of amniotic cells: the two sides of the coin. Cell Transpl. 27, 31–44 (2018).
Lim, R. et al. First-in-human administration of allogeneic amnion cells in premature infants with bronchopulmonary dysplasia: a safety study. Stem Cells Transl. Med. 7, 628–635 (2018).
Acknowledgements
The authors gratefully acknowledge the technical assistance of Ms. Hui Liu and the Monash Histology Research Platform. This study was supported by the National Health and Medical Research Council (NHMRC) project grants (APP1021702 and APP1177699), an NHMRC Centre for Research Excellence (1057514), NHMRC Senior Research Fellowships (J.J.P.: 1077691, T.J.M.: 1043294), an NHMRC CJ Martin Fellowship (R.G.: 1090890) and the Victorian Government’s Operational Infrastructure Support Program.
Author information
Authors and Affiliations
Contributions
R.G., T.J.M. and J.J.P. conceptualized and designed the study. F.N., J.J.P., M.D., J.M., S.B.K., C.M., I.N., R.L., E.M.W., G.J., G.R.P., T.J.M. and R.G. undertook experiments and analyzed data. F.N. undertook immunohistochemistry, cell quantification, analysis and preparation of figures. R.G. provided overall oversight of the research. All authors critically reviewed the manuscript and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nott, F., Jane Pillow, J., Dahl, M. et al. Brain inflammation and injury at 48 h is not altered by human amnion epithelial cells in ventilated preterm lambs. Pediatr Res 88, 27–37 (2020). https://doi.org/10.1038/s41390-020-0815-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0815-8
This article is cited by
-
Interleukin-1 blockade attenuates white matter inflammation and oligodendrocyte loss after progressive systemic lipopolysaccharide exposure in near-term fetal sheep
Journal of Neuroinflammation (2021)
-
Budesonide with surfactant decreases systemic responses in mechanically ventilated preterm lambs exposed to fetal intra-amniotic lipopolysaccharide
Pediatric Research (2021)