Abstract
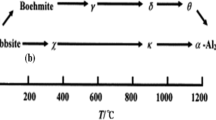
The relationship between ζ-potential and surface defects of micro-nano sized hexagram-like α-Al2O3 particles was investigated in this paper. The 2D α-Al2O3 particles was prepared by the phase transformation of gibbsite precursor by the following steps during calcination in air: Al(OH)3 → AlOOH→a-Al2O3 → θ-Al2O3 → α-Al2O3. Resulting from the varied intensities of surface defects, as-prepared α-Al2O3 samples have different Eg values around 4.5 eV, which is much narrower than that of bulk α-Al2O3. While benefiting from the interface reaction between surface > AlOH0 and water (>AlOH0 + OH−⇋ > AlO− + H2O), as-prepared α-Al2O3 suspensions can reach their specific pHE values after stirring for 8 h at room temperature. And the measured IEP values of Al2O3-1250°C-2 h, Al2O3-1250°C-3 h, and Al2O3–1300 °C-3 h, which remain 2D morphology, are 7.96, 8.67, and 8.86, respectively. This study is significant and useful for making the industrial applications of α-Al2O3 valid.










Similar content being viewed by others
References
Bhattacharyya S, Behera PS (2017) Synthesis and characterization of nano-sized α-alumina powder from kaolin by acid leaching process. Appl Clay Sci 146:286–290
Bodišová K, Galusek D, Švančárek P, Pouchlý V, Maca K (2015) Grain growth suppression in alumina via doping and two-step sintering. Ceram Int 41:11975–11983
Bootkul D, Jitsopakul P, Intarasiri S, Boonyawan D (2017) Qualifying ultrathin alumina film on plasma-enhance atomic layer deposition under low temperature operation. Thin Solid Films 640:116–122
Buchold DHM, Feldmann C (2007) Nanoscale γ-AlO(OH) hollow spheres: synthesis and container-type functionality. Nano Lett 7(11):3489–3492
Cava S, Tebcherani SM, Souza IA, Pianaro SA, Paskocimas CA, Longo E, Varela JA (2007) Structural characterization of phase transition of Al2O3 nanopowders obtained by polymeric precursor method. Mater Chem Phys 103:394–399
Crundwell FK (2016) The mechanism of dissolution of minerals in acdic and alkaline solutions: part V surface charge and zeta potential. Hydrometallurgy 161:174–181
Du X, Li H, Yu J, Xiao X, Shi Z, Mao D, Lu G (2015) Realization of a highly effective Pd-cu-Clx/Al2O3 catalyst for low temperature CO oxidation by pre-synthesizing the active copper phase of Cu2Cl(OH)3. Catal Sci Technol 5:3970–3979
Gangwar J, Gupta BK, Kumar P, Tripathi SK, Srivastava AK (2014) Time-resolved and photoluminescence spectroscopy of θ-Al2O3 nanowires for promising fast optical sensor applications. Dalton Trans 45:17034–17043
Gangwar J, Gupta BK, Tripathi SK, Srivastava AK (2015) Phase dependent thermal and spectroscopic responses of Al2O3 nanostructures with different morphologies. Nanoscale 7:13314–13344
Hai C, Zhou Y, Du Y, Sun Y, Zeng J, Shen Y, Ren X, Li X, Zhang L, Dong O (2017a) Synthesis of MgO nanocrystals with abundant surface defects via a carbonization method employing CO2 gas as starting material. Mater Res Bull 85:181–187
Hai C, Zhou Y, Zhang L, Sun Y, Li X, Shen Y, Zhan H, Han Q, Liu J, Ren H (2017b) Large-scale synthesis of uniformly dispersed hexgram-like gibbsite by a controlled replacement reaction. CrystEngComm 19:3850–3855
Hai C, Zhang L, Zhou Y, Ren X, Liu J, Zeng J, Ren H (2018) Phase transformation and morphology evolution characteristics of hydrothermally prepared boehmite particles. J Inorg Organomet P 28:643–650
Khadzhiev SN, Kadiev KM, Yampolskaya GP, Kadieva MK (2013) Trends in the synthesis of metal oxide nanoparticles through reverse microemulsions in hydrocarbon media. Adv Colloid Interf Sci 197-198:132–145
Kim T, Lian J, Ma J, Duan X, Zheng W (2010) Morphology controllable synthesis of γ-alumina nanostructures via an ionic liquid-assisted hydrothermal route. Cryst Growth Des 20(7):2928–2933
Kumar A, Kumar J (2008) On the synthesis and optical absorption studies of nano-size magnesium oxide powder. J Phys Chem Solids 69:2764–2772
Lee HM, Huang CY, Wang CJ (2009) Forming and sintering behaviors of commercials α-Al2O3 powders with different particle size distribution and agglomeration. J Mater Process Technol 209:714–722
Lee T, Kim W, Lee Y, Ryou M, Lee Y (2014) Effect of Al2O3 coating prepared by RF sputtering on polyethylene separators for high-power lithium ion batteries. Macromol Res 22(11):1190–1195
Liang L, Wang L, Nguyen AV, Xie G (2017) Heterocoagulation of alumina and quartz studied by zeta potential distribution and particle size distribution measurements. Powder Technol 309:1–12
Ma J, Wu B (2013) Effect of surfactants on preparation of nanoscale α-Al2O3 powder by oil-in-water microemulsion. Ceram Int 24:354–358
Mahrouqi DA, Vinogradov J, Jackson MD (2017) Zeta potential of artificial and nutral calcite in aqueous solution. Adv Colloid Interf Sci 240:60–76
Meshigawari K, Mali SS, Sathyamoorthy R, Patil PS (2013) Template-free synthesis of MgO nanoparticles for effective photocatalytic applications. Powder Technol 249:456–462
Nagabhushina KR, Lakshminarasappa BN, Singh F (2009) Photoluminescene and Raman studies in swift heavy ion irradiated polycrystalline aluminum oxide. Bull Mater Sci 32:515–519
Oarida KM, Pradhan AC, Das J, Sahu N (2009) Synthesis and characterization of nano-sized porous gamma-alumina by control precipitation method. Mater Chem Phys 113(1):244–248
Pavan C, Turci F, Tomatis M, Ghiazza M (2017) Zeta potential evidence silanol heterogeneity induced by metal contaminants at the quartz surface: implications in membrane damage. Colloids Surf B: Biointerfaces 157:449–455
Peng H, Liang R, Qiu J (2011) Facile synthesis of Fe3O4@Al2O3 core-shell nanoparticles and their application to the highly specific capture of heme proteins for direct electrochemistry. Biosens Bioelectron 26:3005–3011
Raj SS, Gupta SK, Pathak N, Grover V, Tyagi AK (2017) Origin of visible photoluminescence in combustion synthesized α-Al2O3: Photoluminescence and EPR spectroscopy. Adv Powder Technol 28:1505–1510
Rannabauer S, Sӧffker G, Scheunemann M, Betke U, Scheffler M (2017) Increased mechanical stability and thermal conductivity of alumina reticulated porous ceramics (RPC) by nanoparticle infiltration processing. Adv Eng Mater 19(10):1700211/1–1700211/9
Rao CN, Ramakrishna Matte HS, Rakesh V, Govindaraj (2012) Recent progress in the synthesis of inorganic nanoparticles. Dalton Trans 41:5089–5120
Sharma PK, Varadan VV, Varadan VK (2003) A critical role of pH in the colloidal synthesis and phase transformation of nano size α-Al2O3 with high surface area. J Eur Ceram Soc 23:659–666
Vostrikov AA, Fedyaeva ON (2010) Mechanism and kinetics of Al2O3 nanoparticles formation by reaction of bulk Al with H2O and CO2 at sub- and supercritical conditions. J Supercrit Fluids 55:307–315
Wang N, Hsu C, Zhu L, Tseng S, Hsu J (2013) Influence of metal oxide nanoparticles concentration on their zeta potential. J Colloid Interface Sci 407:22–28
Wang W, Zhang K, Yang Y, Liu H, Qiao Z, Luo H (2014) Synthesis of mesoporous Al2O3 with large surface area and large pore diameter by improved precipitation method. Microporous Mesoporous Mater 193:47–53
Xie W, Sun Y, Liu H (2017) Atomistic investigation on the detachment of oil molecules from defective alumina surface. Appl Surf Sci 426:504–513
Zaki T, Kabel KI, Hassan H (2012) Preparation of high pure α-Al2O3 nanoparticles at low temperatures using Pechini method. Ceram Int 38:2021–2026
Zhang R, Kaliaguine S (2008) Lean reduction of NO by C3H6 over Ag/alumina derived from Al2O3, AlOOH and Al(OH)3. Appl Catal B Environ 78:275–278
Zhang Y, Hu Y, Zhang Y, Liu S (2018) TiO2/void/porous Al2O3 shell embedded in polyvinylidene fluoride film for cleaning wastewater. Adv Powder Technol 29:1582–1590
Funding
We gratefully thank the financially support from the Qinghai Science &Technology Projects (2016-GX-102), the Youth Innovation Promotion Association CAS (2016376), CAS “light of West China” program, the Hundred-Talent Program (Chinese academy of Sciences) and the Qinghai Thousand Talents Program.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hai, C., Zhang, G., Liu, J. et al. ζ-potential variations of micro-nano sized hexagram-like α-Al2O3 particles. J Nanopart Res 22, 65 (2020). https://doi.org/10.1007/s11051-020-04786-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-020-04786-x